Summary
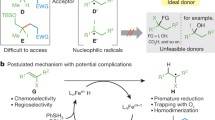
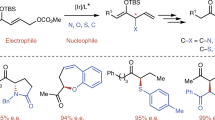
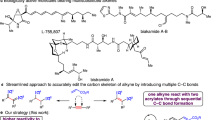
A low molecular weight coumarone (or 2,3-benzofuran)-indene resin was converted to a β-ketoester functional material by Friedel-Crafts acylation with acetyl chloride, reduction to a secondary alcohol and reaction with t-butyl acetoacetate. Each reaction was essentially quantitative as shown by spectroscopy and elemental analysis. The final product proved to be crosslinkable at ambient or elevated temperature by the addition of multifunctional amines.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 5 August 1999/Revised version: 11 January 2000/Accepted: 11 January 2000
Rights and permissions
About this article
Cite this article
Trumbo, D. Functionalization of coumarone-indene resins I. Acetoacetylation. Polymer Bulletin 44, 151–158 (2000). https://doi.org/10.1007/s002890050586
Issue Date:
DOI: https://doi.org/10.1007/s002890050586