Abstract
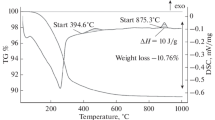
We report here refined values of the transformation enthalpy for xanthoconite to proustite (54.81 kJ/mol) and pyrostilpnite to pyrargyrite (40.32 kJ/mol). Additionally, the enthalpy for the transformation of trechmannite to smithite (5.82 kJ/mol) has been determined. The refinement was possible by taking into account a previously unknown dependence of electrochemical signals on the amount of substance undergoing the reduction process.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received December 21, 1995/Revised, accepted June 29, 1996
Rights and permissions
About this article
Cite this article
Meyer, B., Scholz, F. Redetermination of the transformation enthalpies of the xanthoconite–proustite, pyrostilpnite–pyrargyrite and trechmannite–smithite phase transitions. Phys Chem Min 24, 50–52 (1997). https://doi.org/10.1007/s002690050016
Issue Date:
DOI: https://doi.org/10.1007/s002690050016