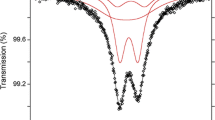
Abstract
Olivine samples (Fa 11) have been oxidized in air (f O2 = 0.2 atm) at temperatures ranging from 350–700 °C and examined by Mössbauer spectroscopy, transmission electron microscopy, X-ray powder diffraction and thermomagnetic analysis. Oxidation of olivine was found to result in ferriolivine, magnesioferrite (major oxide phase) and magnetite (minor oxide phase) formation. Ferriolivine forms planar (001) precipitates, 0.6 nm in thickness, in the olivine host; the composition is likely to be Mg0.5 v 0.5(Fe3+)1.0SiO4. Magnesioferrite MgFe2O4 exsolves as fine-grained precipitates (5–6 nm in size) filling interstices between the ferriolivine planar precipitates. Oxidation kinetic data at 700 °C show two stages of oxidation corresponding to formation of ferriolivine in the first stage and magnesioferrite in the second stage. The linear rate law with a rate constant k Fol = 1.23 · 10-3 s-1 was found for the first stage whereas a parabolic rate-law with a constant of k oxi = 3.28 · 10-3 s-1 was determined for the second stage of oxidation. It was found that ferriolivine is not an intermediate metastable phase in the oxidation process, terminated by magnesioferrite formation. The ferriolivine and magnesioferrite are considered to have formed by independent reactions which do not necessarily proceed simultaneously.
Similar content being viewed by others
References
Banfield IF, Veblen DR, Jones BF (1990) Transmission electron microscopy of subsolidus oxidation and weathering of olivine. Contrib Mineral Petrol 106:110–123
Banfield IF, Dyar MD, McGuire AV (1992) The defect microstructure of oxidized mantle olivine from Dish Hill, California. Amer Miner 77:977–986
Champness PE (1970) Nucleation and growth of iron oxides in olivines, (Mg,Fe)2SiO4 Mineralogical Mag 37:790–800
Dieckman R, Schmalzried H, Mason TO (1981) Kinetics of dense magnetite formation during oxidation of wustite and reduction of hematite in CO/CO2 gas mixture. Arch Eisenhüttenwes 52:211–218
Haggerty SE, Baker I (1967) The alteration of olivine in basaltic and associated lavas. Part I: High temperature alteration. Contrib Mineral Petrol 16:233–257
Hammond PA, Taylor LA (1982) The ilmenite/titano-magnetite assemblage: Kinetics of re-equilibration. Earth and Planetary Sci Letters 61:143–150
Iishi K, Kadomi M, Okamoto K (1989) Synthesis of laihunite by heating Fe — Mn olivine in air. Neues Jahrb Mineral Monatsh 6:245–254
Iishi K, Okamoto K, Kadomi M (1989) Formation of laihunite from Fe — (Mg,Co,Mn,Ca) olivines. Neues Jahrb Mineral Monatsh 8:345–356
Ivanov AP, Safroshkin VY, Trukhin VI, Nekrasov AN (1992) Spectral thermomagnetic analysis of rocks. Izv RAN, Phizika Zemli (in Russian) 3:62–71
Kan X, Coey IMD (1985) Mossbauer spectra, magnetic and electrical properties of laihunite, a mixed valence iron olivine mineral. Amer Miner 70:576–580
Khisina NR, Khramov DA, Kolosov MV, Meshalkin SS (1992) Products of low-temperature oxidation of olivine Mg1.78Fe0.22SiO4. Doklady RAN (in Russian) 324:866–870
Kitamura M, Shen B, Banno S, Morimoto N (1984) Fine textures of laihunite, a nonstoichiometric distorted olivine-type mineral. Amer Miner 69:154–160
Kohlstedt DL, Vander Sande IB (1975) An electron microscopy study of naturally occuring oxidation produced precipitates in iron-bearing olivines. Contrib Mineral Petrol 53:13–24
Koltermann M (1962) Der thermische Zerfall fayalithaltiger Olivine bei hohen Temperaturen. N Jahrb Mineral Monatsh 181–191
Kondoh S, Kitamura M, Morimoto N (1985) Synthetic laihunite (v xFe2 — 3x Fe2x SiO4), an oxidation product of olivine. Amer Miner 70:737–746
Laihunite Research Group (1976) Laihunite — a new iron silicate mineral. Geochimica 2:95–103
Luecke W, Kohlstedt DL (1988) Kinetics of the internal oxidation of (Mg,Fe)O solid solutions. J Am Ceram Soc 71:189–196
Mackwell SI (1992) Oxidation kinetics of fayalite (Fe2SiO4). Phys Chem Miner 19:220–228
Nagata T (1961) Rock magnetism. Maruzen Company Ltd., Tokio
O'Neil HStC, Annersten H, Virgo D (1992) The temperature dependence of the cation distribution in magnesioferrite (MgFe2O4) from powder XRD structural refinements and Mossbauer spectroscopy. Amer Miner 77:725–740
Putnis A (1979) Electron petrografy on high-temperature oxidation of olivine from the Rhum layered intrusion. Mineralogical Mag 43:293–296
Schaefer MW (1983) Measurements of iron (III)-rich fayalites. Nature 303:325–327
Schmalzried H (1983) Internal and external oxidation of nonmetallic compounds and solid solutions (I). Ber Bunsenges Phys Chem 87:551–558
Schwab RB, Kustner D (1977) Präzisionsgitterkonstantenbestimmung zur Festlegung röntgenographischer Bestimmungskurven für synthetische Olivine der Mischkristallreihe Forsterit-Fayalit. N Jahrb Mineral Monatsh 205–215
Tamada O, Shen B, Morimoto N (1983) The crystal structure of laihunite (□0.4Fe 2+0.8 Fe 3+0.8 SiO4). Mineralogical Journal 11:382–391
Veblen DR (1991) Polysomatism and polysomatic series: a review and applications. Amer Miner 76:801–826
Wu T, Kohlstedt DL (1988) Rutherford backscattering spectroscopy study of (Mg, Fe)2SiO4. J Am Ceram Soc 71:540–545
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Khisina, N.R., Khramov, D.A., Kolosov, M.V. et al. Formation of ferriolivine and magnesioferrite from Mg — Fe-olivine: Reactions and kinetics of oxidation. Phys Chem Minerals 22, 241–250 (1995). https://doi.org/10.1007/BF00202257
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00202257