Abstract
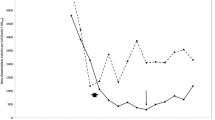
The molar yield coefficients (Y glucose, Y O 2) of glucose-limited continuous cultures of the thermoacidophile Bacillus acidocaldarius have been measured as a function of dilution rate as well as over a range of temperature and pH (51°C to 64°C, pH 2.8–5.5) at a fixed dilution rate of approximately 0.1 h-1. The highest growth yields were observed at 51°C and pH>4.3 (Y glucose 54.8 g cells · mol glucose-1, Y O 2 15.0 g cells · mol O -12 ), but were very much lower than those of mesophilic neutrophiles of similar respiratory chain composition to B. acidocaldarius. Even lower growth yields were observed when the temperature was raised or when the pH was lowered, lowest yields occurring at 64°C and pH 2.8 (Y glucose 23.4 g cells · mol glucose-1, Y O 2 5.9 g cells · mol O -12 ).
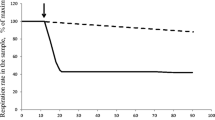
These decreases in growth yield could be correlated with increases in the permeability of the cytoplasmic membrane to protons, i.e. cells needed to catalyse enhanced rates of substrate oxidation in order to avoid a potentially lethal acidification of the cytoplasm. This strategy appears to be successful in that the specific death rates in situ were very low for all cultures except those growing under the most extreme conditions (64°C, pH 2.8).
Similar content being viewed by others
Abbreviations
- FCCP:
-
carbonyl cyanide p-trifluoromethoxy phenylhydrazone
- TMPD:
-
N,N,N′,N′-tetramethyl-p-phenylene diamine
- MES:
-
2-[N-morpholinoðhane sulphonic acid
References
Chicken E, Spode JA, Jones CW (1981) Respiration-linked proton translocation in the moderate thermophile Bacillus stearothermophilus. FEMS Microbiol Lett 11:181–185
Darland G, Brock TD (1971) Bacillus acidocaldarius sp. nov., an acidophilic, thermophilic spore-forming bacterium. J Gen Microbiol 67:9–15
DeRosa M, Gambacorta A, Minale L, Bu'Lock JD (1973) Isoprenoids of Bacillus acidocaldarius. Phytochem 12:1117–1123
Drozd JW, Linton JD, Downs J, Stephenson RJ (1978) An in situ assessment of the specific lysis rate in continuous cultures of Methylococcus sp. (NC1B 11083) grown on methane. FEMS Microbiol Lett 4:311–314
Epstein I, Grossowicz N (1969) Intracellular protein breakdown in a thermophile. J Bacteriol 99:418–421
Farmer IS, Jones CW (1976) The effect of temperature on the molar growth yield and maintenance requirement of Escherichia coli W during aerobic growth in continous culture. FEBS Lett 67:359–363
Farrand SG, Linton JD, Stephenson RJ, McCarthy WV (1983) The use of response surface analysis to study the growth of Bacillus acidocaldarius throughout the growth range of temperature and pH. Arch Microbiol 135:272–275
Guffanti AA, Davidson LF, Mann TM, Krulwich TA (1979) Nigericininduced death of an acidophilic bacterium. J Gen Microbiol 114:201–206
Herbert D, Phipps PJ, Strange RE (1971) Chemical analysis of microbial cells. In: Norris JR, Ribbons DW (eds) Methods in microbiology, vol 1. Academic Press, London New York, pp 209–344
Jones CW, Brice JM, Downs AJ, Drozd JW (1975) Bacterial respiration-linked proton translocation and its relationship to respiratory chain composition. Eur J Biochem 52:265–271
Jones CW (1977) Aerobic respiratory systems in bacteria. In: Haddock BA, Hamilton WA (eds) Microbial energetics. Cambridge University Press, Cambridge, Soc Gen Microbiol Symp 27:23–59
Jones CW, Brice JM, Edwards C (1977) The effect of respiratory chain composition on the growth efficiencies of aerobic bacteria. Arch Microbiol 115:85–93
Kenkel T, Trela JM (1979) Protein turnover in the extreme thermophile Thermus aquaticus. J Bacteriol 140:543–546
Knowles CJ (1977) Microbial metabolic regulation by adenine nucleotide pools. In: Haddock BA, Hamilton WA (eds) Microbial energetics. Cambridge University Press, Cambridge, Soc Gen Microbiol Symp 27:241–283
Kovacs N (1956) Identification of Pseudomonas pyocyanea by the oxidase reaction. Nature (Lond) 178:703
Krulwich TA, Davidson LF, Filip SJ, Zuckerman RS, Guffanti AA (1978) The protomotive force and β-galactoside transport in Bacillus acidocaldarius. J Biol Chem 253:4599–4603
Kuhn HJ, Cometta S, Fiechter A (1980) Effects of growth temperature on maximal specific growth rate, yield, maintenance and death rate in glucose-limited continuous culture of the thermophilic Bacillus caldotenax. Eur J Appl Microbiol Biotechnol 10:303–315
Langworthy TA (1978) Membranes and lipids of extremely thermoacidophilic microorganisms. In: Friedman SM (ed) Biochemistry of thermophily. Academic Press, New York San Francisco London, pp 11–30
Langworthy TA (1979) Membrane structure of thermocidophilic bacteria. In: Shilo M (ed) Strategies of microbial life in extreme environments. Dahlem Konferenzen, vol. 14. Verlag Chemie, Weinheim, pp 417–432
Light PA, Garland PB (1971) A comparison of mitochondria from Torulopsis utilis grown in continuous culture with glycerol, iron, ammonium, magnesium or phosphate as the growth-limiting nutrient. Biochem J 124:123–134
Linton JD, Buckee JC (1977) Interactions in a methane-utilising mixed culture in a chemostat. J Gen Microbiol 101:219–225
Linton JD, Griffiths K, Gregory M (1981) The effect of mixtures of glucose and formate on the yield and respiration of a chemostat culture of Beneckea natriegens. Arch Microbiol 129:119–122
Linton JD, Harrison DEF, Bull AT (1975) Molar growth yields respiration and cytochrome patterns of Beneckea natriegens when grown at different medium dissolved-oxygen tensions. J Gen Microbiol 90:237–246
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mainzer SE, Hempfling WP (1976) Effects of growth temperature on growth yield and maintenance during glucose-limited continuous culture of Escherichia coli. J Bacteriol 126:251–256
Matsche NF, Andrews JF (1973) A mathematical model for the continuous cultivation of thermophilic microorganisms. In: Sikyta B, Prokop A, Novak M (eds) Advances in microbial engineering, part 1. John Wiley and Sons, New York, Biotechnol Bioeng Symp 4:77–90
McKay A, Quilter J, Jones CW (1982) Energy conservation in the extreme thermophile Thermus thermophilus HB8. Arch Microbiol 131:43–50
Mitchell P (1966) Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev 41:445–502
Neijssel OM, Tempest DW (1975) The regulation of carbohydrate metabolism in Klebsiella aerogenes NCTC 418 organisms growing in chemostat culture. Arch Microbiol 106:251–258
Neijssel OM, Tempest DW (1976) Bioenergetic aspects of aerobic growth of Klebsiella aerogenes NCTC 418 in carbon-limited and carbon sufficient chemostat culture. Arch Microbiol 107:215–221
Oshima T, Arakawa H, Baba M (1977) Biochemical studies on an acidophilic thermophilic bacterium Bacillus acidocaldarius: Isolation of bacteria, intracellular pH and stabilities of biopolymers. J Biochem 81:1107–1113
Pirt SJ (1965) The maintenance energy of bacteria in growing cultures. Proc Roy Soc London Series B 163:224–231
Poole RK, Haddock BA (1975) Effects of sulphate-limited growth in continuous culture on the electron-transport chain and energy conservation in Escherichia coli K12. Biochem J 152:537–546
Scholes P, Mitchell P (1970) Acid-base titration across the plasma membrane of Micrococcus denitrificans: Factors affecting the effective proton conductance and the respiratory rate. J Bioenerget 1:61–72
Stackebrandt E, Woese CR (1981) The evolution of prokaryotes. In: Carlile MJ, Collins JF, Moseley BEB (eds) Molecular and cellular aspects of microbial evolution. Cambridge University Press, Cambridge. Soc Gen Microbiol Symp 32:1–31
Tempest DW (1978) The biochemical significance of microbial growth yields: a reassessemnt. Trends Biochem Sci 3:180–184
Tempest DW, Neijssel OM (1980) Growth yield values in relation to respiration. In: Knowles CJ (ed) Diversity of bacterial respiratory systems, vol 1. CRC Press Ino, Boca Raton, Florida, pp 1–31
Topiwala H, Sinclair CG (1971) Temperature relationship in continuous culture. Biotechnol Bioeng 13:795–813
Yamazaki Y, Koyama N, Nosoh Y (1973) On the acidostability of an acidophilic thermophilic bacterium. Biochim Biophys Acta 314:257–260
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Farrand, S.G., Jones, C.W., Linton, J.D. et al. The effect of temperature and pH on the growth efficiency of the thermoacidophilic bacterium Bacillus acidocaldarius in continuous culture. Arch. Microbiol. 135, 276–283 (1983). https://doi.org/10.1007/BF00413481
Issue Date:
DOI: https://doi.org/10.1007/BF00413481