Abstract
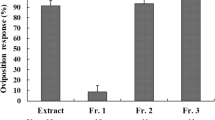
Host-plant chemicals stimulating oviposition by a Leguminosae-feeding pierid butterflyColias erate poliographyswere isolated and identified from one of its primary host plants, white clover (Trifolium repens). Females readily deposited eggs in response to methanolic extracts of the plant, and subsequent partition of the extracts with organic solvents revealed that chemical constituents critical for host recognition reside in the water-soluble fraction. Further fractionation of the hydrosoluble fraction by column chromatography led to the separation of an active fraction and two cyanoglucosides, linamarin and lotaustralin. Conspicuous oviposition response was evoked by unidentified polar compound(s), while these cyanoglucosides exerted no stimulatory activity by themselves. However, ovipositing females preferred samples containing either of the two cyanoglucosides. In dual-choice bioassays, significantly more eggs were laid on samples admixed with the cyanoglucosides, suggesting that the cyanoglucosides serve as synergistic oviposition stimulants and could play an important role in host selection.
Similar content being viewed by others
REFERENCES
BRATTSTENL. B.SAMUELIANJ. H.LONGK. Y.KINCAIDS. A., and EVANSC. K. 1983. Cyanide as a feeding stimulant for the southern armywormSpodoptera eridiana. Ecol. Entomol. 8:125–132.
BUTLERG. W. 1965. The distribution of the cyanoglucosides linamarin and lotaustralin in higher plants. Phytochemistry 4:127–131.
CHEWF. S., and RENWICKJ. A. A. 1995. Host-plant choice in Pieris butterflies, pp. 214–238in R. T. Cardé, and W. J. Bell (eds.). Chemical Ecology of Insects 2. Chapman & Hall, New York.
COMPTONS. G., and JONESD. A. 1985. An investigation of the responses of herbivores to cyanogenesis in Lotus corniculatus L. Biol. J. Linn. Soc. 26:21–38.
DUFFEYS. S. 1981. Cyanide in arthropods, pp. 385–414in B. Vennesland, E. E. Conn, C. Knowles, J. Westley, and F. Wissing (eds.). Cyanide in Biology. Academic Press, London.
ENDOS., and NIHIRAI. 1990. Larval Food of Japanese Butterflies. Group Tamamushi, Tokyo, 74 pp.
FEENYP. 1992. The evolution of chemical ecology: Contributions from the study of herbivorous insects, pp. 1–44in G. A. Rosenthal, and M. R. Berenbaum (eds.). Herbivores: Their Interactions with Secondary Plant Metabolites; Vol. II, Ecological and Evolutionary Processes, Academic Press, San Diego.
FEENYP.SACHDEV-GUPTAK.ROSENBERRYL., and CARTERM. 1988. Luteolin 7-O-(6″-O-malonyl)-β-D-glucoside and trans-chlorogenic acid: Oviposition stimulants for the black swallowtail butterfly. Phytochemistry 27:3439–3448.
HONDAK. 1990. Identification of host-plant chemicals stimulating oviposition by swallowtail butterflyPapilio protenor. J. Chem. Ecol. 16:325–337.
HONDAK. 1995a. Chemical basis of differential oviposition by lepidopterous insects. Arch. Insect Biochem. Physiol. 30:1–23.
HONDAK. 1995b. Plant secondary metabolites implicated for host selection and host specificity in butterflies. Comp. Physiol. Biochem. 12:145–165.
HONDAK.TADAA.HAYASHIN.ABEF., and YAMAUCHIT. 1995. Alkaloidal oviposition stimulants for a danaid butterflyIdeopsis similis L., from a host plantTylophora tanakae (Asclepiadaceae). Experientia 51:753–756.
HUANGX.RENWICKJ. A. A., and SACHDEV-GUPTAK. 1993. A chemical basis for differential acceptance of Erysimum cheiranthoides by two Pieris species. J. Chem. Ecol. 19:195–210.
HUANGX.RENWICKJ. A. A., and SACHDEV-GUPTAK. 1994. Oviposition stimulants in Barbarea vulgaris for Pieris rapae and P. napi oleracea: Isolation, identification and differential activity. J. Chem. Ecol. 20:423–438.
JONESD. A. 1962. Selective eating of the acyanogenic form of the plant Lotus corniculatus L. by various animals. Nature 193:1109–1110.
JONESD. A. 1988. Cyanogenesis in animal-plant interactions, pp. 151–170in CIBA Foundation (ed.). Cyanide Compounds in Biology. Wiley, Chichester.
NAHRSTEDTA. 1986. Uptake of linamarin and lotaustralin from their foodplant by larvae of Zygaena trifolii. Phytochemistry 25:2299–2302.
NAHRSTEDTA. 1987. Recent developments in chemistry, distribution and biology of the cyanogenic glycosides, pp. 213–234in K. Hostettmann and P. J. Lea (eds.). Biologically Active Natural Products. Clarendon Press, Oxford.
NAHRSTEDTA. 1988. Cyanogenesis and the role of cyanogenic compounds in insects, pp. 131–145in CIBA Foundation (ed.). Cyanide Compounds in Biology. Wiley, Chichester.
NISHIDAR. 1995. Oviposition stimulants of swallowtail butterflies, pp. 17–26in J. M. Scriber, Y. Tsubaki, and R. C. Lederhouse (eds.). Swallowtail Butterflies; Their Ecology and Evolutionary Biology. Scientific Publishers, Gainesville.
NISHIDAR., and FUKAMIH. 1989. Oviposition stimulants of an Aristolochiaceae-feeding swallowtail butterflyAtrophaneura alcinous. J. Chem. Ecol. 15:2565–2575.
NISHIDAR.OHSUGIT.KOKUBOS., and FUKAMIH. 1987. Oviposition stimulants of a Citrus-feeding swallowtail butterflyPapilio xuthus L. Experientia 43:342–344.
OHSUGIT.NISHIDAR., and FUKAMIH. 1991. Multi-component system of oviposition stimulants for a Rutaceae-feeding swallowtail butterflyPapilio xuthus (Lepidoptera: Papilionidae). Appl. Entomol. Zoo. 26:29–40.
PITSCHCh.KELLERM.ZINSMEISTERH. D., and NAHRSTEDTA. 1984. Cyanogenic glycosides from Triticum monococcum. Planta Med. 44:388–390.
RENWICKJ. A. A., and CHEWF. S. 1994. Oviposition behavior in Lepidoptera. Annu. Rev. Entomol. 39:377–400.
SACHDEV-GUPTAK.FEENYP. P., and CARTERM. 1993. Oviposition stimulants for the pipevine swallowtail butterflyBattus philenor (Papilionidae), from an Aristolochia host plant: Synergism between inositols, aristolochic acids and a monogalactosyl diglyceride. Chemoecology 4:19–28.
TRAYNIERR. M. M., and TRUSCOTTR. J. W. 1991. Potent natural egg-laying stimulant for cabbage butterfly Pieris rapae. J. Chem. Ecol. 17:1371–1380. ai]VAN LOONJ. J. A.BLAAKMEERA.GRIEPINKF. C.VAN BEEKT. A.SCHOONHOVENL. M., and DE GROOTA. 1992. Leaf surface compound from Brassica oleracea (Cruciferae) induces oviposition by Pieris brassicae (Lepidoptera: Pieridae). Chemoecology 3:39–44.
WITTHOHNK., and NAUMANNC. M. 1987. Cyanogenesis—a general phenomenon in the Lepidoptera? J. Chem. Ecol. 13:1789–1809.
WRAYV.DAVISR. H., and NAHRSTEDTA. 1983. Biosynthesis of cyanogenic glycosides in butterflies and moths: Incorporation of valine and isoleucine into linamarin and lotaustralin by Zygaena and Heliconius species (Lepidoptera). Z. Naturforsch. 38c:583–588.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Honda, K., Nishii, W. & Hayashi, N. Oviposition Stimulants for Sulfur ButterflyColias erate poliographys: Cyanoglucosides as Synergistis Involved in Host Preference. J Chem Ecol 23, 323–331 (1997). https://doi.org/10.1023/B:JOEC.0000006362.96722.c9
Issue Date:
DOI: https://doi.org/10.1023/B:JOEC.0000006362.96722.c9