Abstract
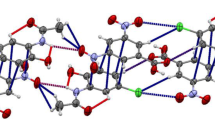
The red colored and poorly soluble 1,6-bis(o-hydroxyanilino)-1,5-hexadiene-3,4-dione (3f) forms stable adducts5 and7 of exact stoichiometric ratios withN,N-dimethyl carboxamides4 and ureas6. The adducts are yellow colored and easily soluble in organic solvents. The crystal structure of3f (monoclinic space groupP2 1/c witha=7.903(2),b=10.941(2),c=8.976(3) Å, β=90.13(2)°) indicates planarity with extensive delocalization of the π-electrons. The poor solubility is referred to the formation of strong intermolecular hydrogen bonds (H(O)...O3 andH(O′)...O′3=1.79 Å). The crystal structure of the 1∶2 DMF adduct5a (monoclinic space groupP2 1/c witha=6.068(1),b=19.668(2),c=10.645(1) Å, β=107.791(8)°) shows a less pronounced delocalization of the π-electrons which might be the explanation for the color change from red to yellow. In5a the intermolecular hydrogen bonds of3f are interrupted by forming new hydrogen bonds from the hydroxyl group to the carbonyl group of DMF (H(O)...O(L) andH(O′)...O(L)=1.86 Å), whereby the solubility is markedly changed. The thermal stability of the addition products5 and7 was determined by thermogravimetry.
Similar content being viewed by others

References
Effenberger, F.Chem. Ber. 1965,98, 2260–2265.
Effenberger, F.; Bonacorso, H. G.; Mack, K.-E.J. Heterocyclic Chem. 1995,32, 57–64.
Barthelmess, I..Part of Dissertation; University of Stuttgart, Germany, 1985.
Rösch, N.Forschungspraktikum; University of Stuttgart, Germany, 1984.
Stewart, J. M.; Dickinson, P. A.; Ammon, H. L.; Flack, H.; Heck, H.Program XRAY-76. Tech. Rept. TR-446, University of Maryland, Computer Center: College Park, MD, 1976.
Rapoport, H.; Bonner, R. M.J. Am. Chem. Soc. 1950,72, 2783–2784.
Trost, B. M.; Kinson, P. L.; Maier, C. A.; Paul, I. C.J. Am. Chem. Soc. 1971,93, 7275–7281.
Allen, F. H..Acta. Crystallogr. 1981, B37, 890–900.
Dewar, M. J. S.; Schmeising, H. N..Tetrahedron 1960,11, 96–120.
Kuchitsu, K.; Fukuyama, T.; Morino, Y..J. Mol Struct. 1967/1968,1, 463–479.
Brown, K. L.; Damm, L.; Dunitz, J. D.; Eschenmoser, A.; Hobi, R.; Kratky, C.Helv Chim. Acta. 1978,61, 3108–3135.
Robin, M. B.; Bovey, F. A.; Basch, H. InThe Chemistry of Amides; Patai, S. Ed.; Interscience Publishers: London, 1970; pp 1–72.
Macdonald, A. L.; Trotter, J.J. Chem. Soc. Perkin Trans. 2 1973, 476–480.
Danielson, D. D.; Hedberg, K.J. Am. Chem. Soc. 1979,101, 3730–3734.
Effenberger, F.; Kramme R.; Lindner, H. J.; Martin, G.; Martin, H.-D.; Mayer, B.Chem. Ber. 1991,124, 827–832.
Kaftory, M.; Rubin, M. B.J. Chem. Soc. Perkin Trans. 2 1983, 149–154.
Lister, D. G.; Tyler, J. K.; Høg, J. H.; Wessel Larsen, N.:J. Mol. Structure 1974,23, 253–264.
Czugler, M.; Stezowski, J. J.; Weber, E.J. Chem. Soc. Chem. Commun. 1983, 154–155.
Ottersen, T.; Rosenqvist, E.Acta. Chem. Scand. 1977, B31, 749–755.
Cobbledick, R. E.; Small, R. W. H.Acta. Crystallogr. 1973, B29, 1659–1666.
Cobbledick, R. E.; Small, R. W. H.Acta. Crystallogr. 1975, B31, 2805–2808.
Brown, J. N.; Agrawal, K. C.Acta. Crystallogr. 1977 B33, 980–984.
Johnson, C. K..A Fortran Thermal Ellipsoid Plot Program for Crystal Structure Illustrations; Tech. Rept. ORNL-5138, Oak Ridge National Laboratory, Oak Ridge, TN, 1971.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Effenberger, F., Barthelmess, I. & Rösch, N. Stable crystalline adducts of 1,6-bis(o-hydroxyanilino)-1,5-hexadiene-3,4-dione withN,N-dimethyl carboxamides and urea derivatives. J Chem Crystallogr 25, 493–498 (1995). https://doi.org/10.1007/BF01665706
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01665706