Abstract
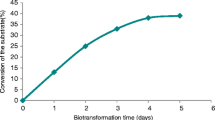
Although alginate-entrapped cells of Mucuna pruriens L. possess a low substrate specificity, only para-substituted monocyclic phenols have been ortho-hydroxylated into catechols so far. In this study, compounds with more complex chemical structures were found to be substrates using entrapped cells of M. pruriens as well as the partially purified Mucuna-phenoloxidase. Thus, 5-, 6- and 7-hydroxylated 2-aminotetralins and a tricyclic compound, 9-hydroxy N-n-propyl hexahydronaphthoxazine, were converted into catechols. After isolation using preparative HPLC, the identity of the products was confirmed by MS. In general, for the entrapped cells and the enzyme preparation identical substrate specificities were found.
Similar content being viewed by others
References
Pras N, Wichers HJ, Bruins AP, Malingré ThM (1988) Bioconversion of para-substituted monophenolic compounds into corresponding catechols by alginate-entrapped cells of Mucuna pruriens. Plant Cell Tissue Organ Culture 13: 15–26
Pras N, Hesselink PGM, Guikema WM, Malingré ThM (1989) Further kinetic characterization of alginate-entrapped cells of Mucuna pruriens L. Biotechnol Bioeng 33: 1461–1468
Pras N, Hesselink PGM, Tusscher Jten, Malingré ThM (1989) Kinetic aspects of the bioconversion of L-tyrosine into L-DOPA by cells of Mucuna pruriens L. entrapped in different matrices. Biotechnol Bioeng 34: 214–222
McDermed JD, McKenzie GM, Philips AP (1975) Synthesis and pharmacology of some 2-aminotetralins: Dopamine receptor agonists. J Med Chem 18: 362–367
Horn AS, Kaptein B, Vermue NA, Vries JBde, Mulder TBA (1988) Synthesis and dopaminergic activity of a new oxygen isostere of the 2-aminotetralins: N,N-dipropyl-8-hydroxy-3-chromanamine. Eur J Med Chem 23: 325–328
Dijkstra D, Hazelhoff B, Mulder TBA, Vries JBde, Wijnberg H, Horn AS (1985) Synthesis and pharmacological activity of the hexahydro-4H-naphth[1,2b][1,4]-oxazines: a new series of potent dopamine receptor agonists. Eur J Med Chem 20: 247–250
Huizing HJ, Wichers HJ (1984) Production of L-DOPA by Mucuna pruriens cell suspension cultures through accumulation or by biotransformation of tyrosine. In: Houwink EH, Meer RRvan der (Eds) Innovations in Biotechnology, Progress in Industrial Microbiology 20 (pp 217–228) Elseviers Science Publishers, Amsterdam
Wiehers HJ, Malingré ThM, Huizing HJ (1983) The effect of some environmental factors on the production of L-DOPA by alginate-entrapped cells of Mucuna pruriens. Planta 158: 482–486
Wichers HJ, Peetsma GJ, Malingré ThM, Huizing HJ (1984) Purification and properties of a phenoloxidase derived from suspension cultures of Mucuna pruriens. Planta 162: 334–341
Bruins AP, Pras N (1984) Isolation of 3,4-dihydroxy-phenylacetic acid produced from p-hydroxyphenylacetic acid by immobilized plant cells of Mucuna pruriens L. and its identification by liquid chromatography/mass spectrometry. Anal Chim Acta 163: 91–101
Abul-Hajj YJ, Cisek PL (1986) Regioselective reaction of thiols with catechol estrogens and estrogen-O-quinones. J Steroid Biochem 25: 245–247
Capdevielle P, Maury M (1982) Ortho-hydroxylation sélective des phenols. I. Vers un modèle chimique simple des tyrosinases. Tetrahydron Lett 25: 1573–1576
Bruins AP (1985) Developments in interfacing microbore high-performance liquid chromatography with mass spectrometry (a review) J Chromatogr 323: 99–111
Author information
Authors and Affiliations
Additional information
This publication is dedicated to the memory of Prof. Alan S. Horn, Ph.D., who deceased at January 2, 1990
Rights and permissions
About this article
Cite this article
Pras, N., Booi, G.E., Dijkstra, D. et al. Bioconversion of bi- and tri-cyclic monophenols by alginate-entrapped cells of Mucuna pruriens L. and by the partially purified Mucuna-phenoloxidase. Plant Cell Tiss Organ Cult 21, 9–15 (1990). https://doi.org/10.1007/BF00034485
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00034485