Summary
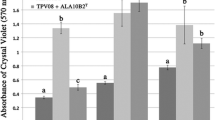
The production of cellulase byRhizobium species was studied.Rhizobium trifolii cellulase was induced by a variety of polysaccharides, including celluloses and hemicelluloses. Cellobiose and myo-inositol also allowed enzyme expression but mannitol prevented it at concentrations higher than 0.25%. Both soluble and insoluble plant root substances moderately stimulated cellulase production byRhizobium trifolii.
Most substances tested did not induce the production of cellulases by the “slow-growing, cowpea type” rhizobia strain CIAT 79. Effective inducers were carboxymethylcellulose, gluconate and myo-inositol.
Cellulase production was very low under all conditions tested. In most cases the enzyme activity was loosely bound to the capsular material. The enzyme in fast-growers is an 1,4-β-D-glucan-4-glucanohydrolase (endo-glucanase EC 3.2.1.4) with specificity for high molecular weight polysaccharides.
There was no correlation between infectiveness ofRhizobium trifolii strains and cellulase production. One strain, which lacks the nodulation plasmid, produced cellulase at the same rate as its parental infective strain.
Similar content being viewed by others
Bibliography
Bergersen F J 1961 The growth of Rhizobium in synthetic media. Aust. J. Biol. Sci. 14, 349–360.
Callham D A and Torrey J G 1981 The structural basis for infection of root hairs ofTrifolium repens by Rhizobium Can. J. Bot. 59, 1647–1664.
Chandler M R 1978 Some observations of infection ofArachis hypogea L. by Rhizobium. J. Exp. Bot. 29, 749–755.
Hodge J E and Hofreiter B T 1962 Determination of reducing sugars and carbohydrates. p. 380–394In Methods in Carbohydrate Chemistry. Eds. R L Whistler and M L Wolfrom. Vol 1 Academic Press, London.
Hooykaas P J J, van Brussel A A N, den Dulk-Ras H, van Slogteren G M S and Schilperoort R A 1981 Sym plasmid ofRhizobium trifolii expressed in different rhizobial species andAgrobacterium tumerfaciens. Nature London 291, 351–353.
Hubbell D H, Morales V M and Umali-Garcia M 1978 Pectolytic enzymes inRhizobium. Appl. Environ. Microbiol. 32, 210–213.
Li D and Hubbell D H 1969 Infection thread formation as a basis of nodulation specificity in Rhizobium-strawberry clover associations. Can. J. Microbiol. 15, 1135–1136.
Lillich T T and Elkan G H 1968 Evidence countering the role of polygalacturonase in invasion of root hairs of leguminous plants byRhizobium spp. Can. J. Microbiol. 14, 617–625.
Ljunggren H and Gahraeus G 1961 The role of polygalacturonase in root-hair invasion by nodule bacteria. J. Gen. Microbiol. 26, 521–528.
Lowry O H, Rosebrough N J, Farr A and Randall R J 1951 Protein measurements with the Folin phenol reagent. J. Biol. Chem. 193, 265–275.
Macmillan J D and Cooke R C 1969 Evidence against involvement of pectic enzymes in the invasion of root hairs byRhizobium trifolii. Can. J. Microbiol. 15, 643–645.
Martinez-Molina E, Morales V M and Hubbell D H 1969 Hydrolytic enzyme production by Rhizobium. Appl. Environ. Microbiol. 38, 1186–1188.
Napoli C A and Hubbell D H 1975 Ultrastructure of Rhizobium-induced infection threads in clover root hairs. Appl. Microbiol. 30, 1003–1009.
Nutman P S 1956 The influence of the legume in root nodule symbiosis: A comparative study of host determinants and functions. Biol. Rev. Cambridge Philos. Soc. 31, 109–151.
Olivares J, Montoya E and Palomares A 1977 Some effects derived from the presence of extrachromosomal DNA inRhizobium meliloti. p. 375–385.In Recent Developments in Nitrogen Fixation. Eds. W Newton, J R Postgate and C Rodriguez-Barrueco. Academic Press, New York.
Palomares A J 1975 Production de poligalacturonasas en la association Rhizobium-leguminosa. Ph. D. Thesis, Universidad de Granada, Granada, Spain, 153 pp.
Solheim B and Raa J 1971 Evidence countering the theory of specific induction of pectindegrading enzymes as the basis for specificity in Rhizobium leguminous associations. Plant and Soil 35, 275–280.
Somogyi M 1952 Notes on sugar determination. J. Biol. Chem. 195, 19–24.
Verma D P S and Zogbi V 1978 A cooperative action of plant and Rhizobium to dissolve the host cell wall during development of root nodule symbiosis. Plant Sci. Letters 13, 137–142.
Vincent J M 1970 A Manual for the Practical Study of Root Nodule Bacteria. Blackwell Sci. Publ., Oxford.
Zurkowski W and Lorkiewicz Z 1979 Plasmid-mddiated control of nodulation inRhizobium trifolii. Arch. Microbiol. 123, 195–201.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Morales, V.M., Martinez-Molina, E. & Hubbell, D.H. Cellulase production by Rhizobium. Plant Soil 80, 407–415 (1984). https://doi.org/10.1007/BF02140047
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02140047