Abstract
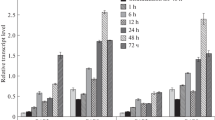
The time course of the accumulation of the transcripts from 13 psb genes encoding a major part of the proteins composing photosystem II during light-induced greening of dark-grown wheat seedlings was examined focusing on early stages of plastid development (0.5 h through 72 h). The 13 genes can be divided into three groups. (1) The psbA gene is transcribed as a single transcript of 1.3 kb in the dark-grown seedlings, but its level increases 5- to 7-fold in response to light due to selective increase in RNA stability as well as in transcription activity. (2) The psbE-F-L-J operon, psbM and psbN genes are transcribed as a single transcript of 1.1 kb, two transcripts of 0.5 and 0.7 kb and a single transcript of 0.3 kb, respectively, in the dark-grown seedlings. The levels of accumulation of every transcript remain unchanged or rather decrease during plastid development under illumination. (3) The psbK-I-D-C gene cluster and psbB-H operon exhibit fairly complicated northern hybridization patterns during the greening process. When a psbC or psbD gene probe was used for northern hybridization, five transcripts differing in length were detected in the etioplasts from 5-day old dark-grown seedlings. After 2 h illumination, two new transcripts of different length appeared. Light induction of new transcripts was also observed in the psbB-H operon.
Similar content being viewed by others
References
Barkan A: Proteins encoded by a complex chloroplast transcription unit are each translated from both monocistronic and polycistronic mRNAs. EMBO J 7: 2637–2644 (1988).
Berends T, Kubicek Q, Mullet E: Localization of the genes coding for the 51 kDa PSII chlorophyll apoprotein, apocytochrome b6, the 65–70 kDa PSI chlorophyll apoproteins and the 44 kDa PSII chlorophyll apoprotein in pea chloroplast DNA. Plant Mol Biol 6: 125–134 (1986).
Berends T, Gamble PE, Mullet JE: Characterization of the barley chloroplast transcription units containing psaA-psaB and psbD-psbC. Nucl Acids Res 15: 5217–5240 (1987).
Chory J, Peto CA: Mutations in the DET1 gene affect cell-type-specific expression of light-regulated genes and chloroplast development in Arabidopsis. Proc Natl Acad Sci USA 87: 8776–8780 (1990).
Chory J, Nagpal P, Peto CA: Phenotypic and genetic analysis of det2, a new mutant that affects light-regulated seedling development in Arabidopsis. Plant Cell 3: 445–459 (1991).
Courtice GRM, Bowman CM, Dyer TA, Gray JC: Localisation of genes for components of photosystem II in chloroplast DNA from pea and wheat. Curr Genet 10: 329–333 (1985).
Cushman JC, Christopher DA, Little MC, Hallick RB, Price CA: Organization of the psbE, psbF, orf38, and orf42 gene loci on the Euglena gracilis chloroplast genome. Curr Genet 13: 173–180 (1988).
Deng XW, Gruissem W: Control of plastid gene expression during development: the limited role of transcriptional regulation. Cell 49: 379–387 (1987).
Fromm H, Devic M, Fluhr R, Edelman M: Control of psbA gene expression: in mature Spirodela chloroplasts light regulation of 32-kd protein synthesis is independent of transcript level. EMBO J 4: 291–295 (1985).
Gamble PE, Sexton TB, Mullet JE: Light-dependent changes in psbD and psbC transcripts of barley chloroplasts: accumulation of two transcripts maintains psbD and psbC translation capability in mature chloroplasts. EMBO J 7: 1289–1297 (1988).
Glick RE, McCauley SW, Gruissem W, Melis A: Light quality regulates expression of chloroplast genes and assembly of photosynthetic complexes. Proc Natl Acad Sci USA 83: 4287–4291 (1986).
Gray JC: Genetics and synthesis of chloroplast membrane proteins. In: Amesz J (ed) Photosynthesis, pp. 319–342. Elsevier, Amsterdam (1987).
Gruissem W: Chloroplast gene expression: How plants turn their plastids on. Cell 56: 161–170 (1989).
Herrmann RG, Westhoff P, Alt J, Tittgen J, Nelson N: Thylakoid membrane proteins and their genes. In: van Vloten-Doting L, Groot GSP, Hall TC (eds) Molecular Form and Function of the Plant Genome, pp. 233–256. Plenum Press, New York (1985).
Hiratsuka J, Shimada H, Whittier R, Ishibashi T, Sakamoto M, Mori M, Kondo C, Honji Y, Sun C-R, Meng B-Y, Li Y-Q, Kanno A, Nishizawa Y, Hirai A, Shinozaki K, Sugiura M: The complete sequence of the rice (Oryza sativa) chloroplast genome: intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mol Gen Genet 217: 185–194 (1989).
Howe CJ, Barker RF, Bowman CM, Dyer TA: Common features of three inversions in wheat chloroplast DNA. Curr Genet 13: 343–349 (1988).
Klein RR, Mullet JE: Control of gene expression during higher plant chloroplast biogenesis: protein synthesis and transcript levels of psbA, psaA-psaB, and rbcL in dark-grown and illuminated barley seedlings. J Biol Chem 242: 4341–4348 (1987).
Klein RR, Mullet JE: Light-induced transcription of chloroplast genes: psbA transcription is differentially enhanced in illuminated barley. J Biol Chem 265: 1895–1902 (1990).
Kohchi T, Yoshida T, Komano T, Ohyama K: Divergent mRNA transcription in the chloroplast psbB operon. EMBO J: 885–891 (1988).
Mullet JE: Chloroplast development and gene expression. Annu Rev Plant Physiol Plant Mol Biol 39: 475–502 (1988).
Obokata J: Synthesis and assembly of the polypeptides of photosystem I and II isolated etiochloroplasts of wheat. Plant Physiol 84: 535–540 (1987).
Ohyama K, Fukuzawa H, Kohchi T, Shirai H, Sano T, Sano S, Umesono K, Shiki Y, Takeuchi M, Chang Z, Aota S, Inokuchi H, Ozeki H: Chloroplast gene organization deduced from complete sequence of liverwort Marchantia polymorpha chloroplast DNA. Nature 322: 572–574 (1986).
Rodelmel SR, Bogorad L: Maize plastid photogenes: mapping and photoregulation of transcript levels during light-induced development. J Cell Biol 100: 436–476 (1985).
Schrubar H, Wanner G, Westhoff P: Transcriptional control of plastid gene expression in greening Sorghum seedlings. Planta 183: 101–111 (1990).
Schuster G, Gruissem W: Chloroplast mRNA 3′ end processing requires a nuclear-encoded RNA-binding protein. EMBO J 10: 1493–1502 (1991).
Sexton TB, Jones JT, Mullet JE: Sequence and transcriptional analysis of the barley ctDNA region upstream of psbD-psbC encoding trnK(UUU), rps16, trnQ(UUG), psbK, psbI, and trnS(GCU). Curr Genet 17: 445–454 (1990).
Shinozaki K, Ohme M, Wakasugi T, Hayashida N, Matsubayashi T, Zaita N, Chunwongse J, Obokata J, Yamaguchi-Shinozaki K, Ohta C, Torazawa K, Meng BY, Sugita M, Deno H, Kamogashira T, Yamada K, Kusuda J, Takaiwa F, Kato A, Tohdoh N, Shimada H, Sugiura M: The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J 5: 2043–2049 (1986).
Sugiura M: The chloroplast chromosomes in land plants. Annu Rev Cell Biol 5: 51–70 (1989).
Tanaka M, Obokata J, Chunwongse J, Shinozaki K, Sugiura M: Rapid splicing and stepwise processing of a transcript from the psbB operon in tobacco chloroplasts: Determination of the intron sites in petB and petD. Mol Gen Genet 209: 427–431 (1987).
Webber AN, Hird SM, Packman LC, Dyer TA, Gray JC: A photosystem II polypeptide is encoded by an open reading frame contranscribed with genes for cytochrome b-559 in wheat chloroplast DNA. Plant Mol Biol 12: 141–151 (1989).
Westhoff P, Herrmann RG: Complex RNA maturation in chloroplasts: The psbB operon from spinach. Eur J Biochem 171: 551–564 (1988).
Willey DL, Gray JC: Two small open reading frames are contranscribed with the pea chloroplast genes for the polypeptides of cytochrome b-559. Curr Genet 15: 213–220 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kawaguchi, H., Fukuda, I., Shiina, T. et al. Dynamical behavior of psb gene transcripts in greening wheat seedlings. I. Time course of accumulation of the psbA through psbN gene transcripts during light-induced greening. Plant Mol Biol 20, 695–704 (1992). https://doi.org/10.1007/BF00046454
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00046454