Abstract
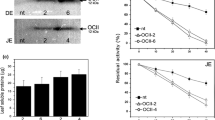
Insecticidal effects of three plant-derived genes, those encoding snowdrop lectin (GNA), bean (Phaseolus vulgaris) chitinase (BCH) and wheat α-amylase (WAI), were investigated and compared with effects of the cowpea trypsin inhibitor gene (CpTI). Transgenic potato plants containing each of the three genes singly, and in pairwise combinations were produced. All the introduced genes were driven by the CaMV 35S promoter; expression was readily detectable at the RNA level in transformants, but not detectable accumulation of WAI could be detected in transgenic potatoes containing its encoding gene. GNA and BCH were accumulated at levels up to 2.0% of total soluble protein; both proteins were expressed in a functional form, and GNA was shown to undergo 'correct' N-terminal processing. Accumulation levels of individual proteins were higher in plants containing a single foreign gene than in plants containing two foreign genes.
Resistance of the transgenic plants to insect attack was assayed by exposing the plants to larvae of the tomato moth, Lacanobia oleracea. All the plants tested which were expressing GNA showed an enhanced level of resistance. Leaf damage was reduced by more than 50% compared to controls; total insect biomass per plant was reduced by 45-65%, but larval survival was only slightly reduced (20%). These results support the hypothesis that GNA has a significant antifeedant effect on insects. Expression of BCH had no protective effect against this insect. Expression of CpTI in transgenic potatoes had similar effects to expression of GNA on total insect biomass and survival, but did not afford protection against insect damage to the plant.
Similar content being viewed by others
References
Barton KA, Miller MJ: Production of Bacillus thuringiensisinsecticidal proteins in plants. In: Kung S, Wu R (eds) Transgenic Plants, vol. 1, pp. 297–315. Academic Press, New York (1993).
Barton KA, Whiteley H, Yang N: Bacillus thuringiensisendotoxin in transgenic Nicotiana tabacumprovides resistance to lepidopteran insects. Plant Physiol 85: 1103–1109 (1987).
Boulter D, Edwards GA, Gatehouse AMR, Gatehouse JA, Hilder VA: Additive protective effects of different plant-derived insect resistance genes in transgenic tobacco plants. Crop Prot 9: 351–354 (1990).
Broglie KE, Gaynor JJ, Broglie RM: Ethylene-regulated gene expression. In: Molecular cloning of the genes encoding an endochitinase from Phaseolus vulgaris.Proc Natl Acad Sci USA 83: 6820–6824 (1986).
Czapla TH, Lang BA: Effect of plant lectins on the larval development of European corn borer (Lepidoptera: Pyralidae) and Southern corn rootworm (Coleoptera: Chrysomelidae). J. Econ Entomol 83: 2480–2485 (1990).
Ditta G, Stanfield S, Corbin D, Helinski DR: Broad host range DNA cloning system for Gram-negative bacteria: construction of a gene bank of Rhizobium meliloti.Proc Natl Acad Sci USA 77: 7347–7351 (1980).
Edmonds HS: Antimetabolic proteins from plants and their potential use in conferring resistance against corn rootworms (Diabroticasp.) Ph. D. thesis, University of Durham (1994).
Eisemann CH, Donaldson RA, Pearson RD, Cadogan LC, Vuocolo T, Tellam RL: Larvicidal activity of lectins on Lucilia cuprina:mechanism of action. Entomol Exp Appl 72: 1–10 (1994).
Ellis JR, Shirsat AH, Hepher A, Yarwood JN, Gatehouse JA, Croy RRD, Boulter D: Tissue specific expression of a pea legumin gene in seeds of Nicotiana plumbaginifolia.Plant Mol Biol 10: 203–214 (1988).
Feinberg AP, Vogelstein B: Atechnique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132: 6–13 (1983).
Gamborg OL, Miller RA, Ojima K: Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50: 151–158 (1968).
Gatehouse AMR, Dewey FM, Dove J, Fenton KA, Pusztai, A: Effect of seed lectin from Phaseolus vulgarison the development of larvae of Callosobruchus maculatus:mechanism of toxicity. J Sci Food Agric 35: 373–380 (1984).
Gatehouse AMR, Fenton KA, Jepson I, Pavey DJ: The effects of α-amylase inhibitors on insect storage pests: inhibition of α-amylase in vitroand effects on development in vitro.J Sci Food Agric 37: 727–734 (1986).
Gatehouse AMR, Shackley SJ, Fenton KA, Bryden J, Pusztai A: Mechanismof seed lectin tolerance by amajor insect storage pest of Phaseolus vulgaris, Acanthoscelides obtectus.J Sci Food Agric 47: 269–280 (1989).
Gatehouse AMR, Boulter D, Hilder, VA: Potential of plant-derived genes in the genetic manipulation of crops for insect resistance. In: Gatehouse AMR, Hilder VA, Boulter D (eds) Genetic Manipulation for Crop. Protection, pp. 35–153. CAB International, Wallingford (1992).
Gatehouse AMR, Shi Y, Powell KS, Brough C, Hilder VA, Hamilton WDO, Newell CA, Boulter D, Gatehouse JA: Approaches to insect resistance using transgenic plants. Phil Trans R Soc Lond B 342: 279–286 (1993).
Gatehouse AMR, Hilder VA: Genetic manipulation of crops for insect resistance. In: Marshall G, Walters DR (eds) Molecular Perspectives in Crop Protection, pp. 177–201. Chapman and Hall, London (1994).
Gatehouse AMR, Powell KS, Peumans WJ, Van Damme EJM, Gatehouse JA: Insecticidal properties of lectins: their potential in plant protection. In: Pusztai AJ, Bardocz S (eds) Lectins: Biomedical Perspectives, pp. 35–57. Taylor and Francis, Hants, UK (1995).
Gatehouse AMR, Down RE, Powell KS, Newell CA, Hamilton WDO, Gatehouse JA: Transgenic potato plants with enhanced resistance to the peach-potato aphid Myzus persicae.Entomol Exp Appl 79: 295–307 (1996).
Gutierrez C, Garcia Casado G, Sanchez Monge R, Gomez L, Castanera P, Salcedo G: Three inhibitor types from wheat endosperm are differentially active against alpha-amylases of lepidopteran pests. Entomol Exp Appl 66: 47–52 (1993).
Habibi J, Backus EA, Czalpa, TM: Plant lectins affect survival of the potato leafhopper (Homoptera: Cicadellidae). J Econ Entomol 86: 945–951 (1993).
Hilder VA, Gatehouse AMR, Sherman SE, Barker RF, Boulter D: A novel mechanism of insect resistance engineered into tobacco. Nature 330: 160–163 (1987).
Ishimoto M, Kitamura K: Identification of the growth inhibitor on azuki bean weevil in kidney bean (Phaseolus vulgaris). Jpn J Breed 38: 367–370 (1988).
Jefferson RA: Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5: 387–405 (1987).
Johnson R, Narvaez J, An G, Ryan, C: Expression of proteinase inhibitors I and II in transgenic tobacco plants: effects on natural defence against larvae of Manduca sexta.Proc Natl Acad Sci USA 86: 9871–9875 (1989).
Kashlan N, Richardson M: The complete amino acid sequence of a major wheat protein inhibitor of α-amylase. Phytochemistry 20: 1781–1784 (1981).
King TP, Putsztai A, Clarke EMW: Immunocytochemical localisation of ingested kidney bean (Phaseolus vulgaris) lectins in rat gut. Histochem J 12: 201–208 (1980).
Lis H, Sharon N: The biochemistry of plant lectins (phytohaemagglutinins). Annu Rev Biochem 42: 541–574 (1973).
McManus MT, White DWR, McGregor PG: 1994. Accumulation of a chymotrypsin inhibitor in transgenic tobacco can affect the growth of insect pests. Transgen Res 3: 50–58 (1994).
Murashige T, Skoog F: A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473–497 (1962).
Newell CA, Rozman R, Hinchee MA, Lawson EC, Haley L, Sanders P, Kaniewski W, Tumer NE, Horsch RB, Fraley RT: Agrobacterium-mediated transformation of Solanum tuberosumL. cv. Russet Burbank. Plant Cell Rep 10: 30–34 (1991).
Ooms G, Hooykaas PJJ, van Veen RJM, van Beelen P, Regensburg-Tuink, Schilperoort RA: Octopine Ti-plasmid deletion mutants of Agrobacterium tumefacienswith emphasis on the right side of the T-region. Plasmid 7: 15–29 (1982).
Peferoen M: Engineering of insect-resistant plants with Bacillus thuringiensiscrystal protein genes. In: Gatehouse AMR, Hilder VA, Boulter D (eds) Plant Genetic manipulation for Crop Protection, pp. 135–153. CAB International, Wallingford (1992).
Perlak FJ, Deaton RW, Armstrong TA, Fuchs RL, Sims SR, Greenplate JT, Fischhoff DA: Insect resistant cotton plants. Bio/technology 8: 939–943 (1990).
Powell KS, Gatehouse AMR, Hilder VA, Gatehouse JA: Anti-metabolic effects of plant lectins and plant and fungal enzymes on the nymphal stages of two important rice pests, Nilaparvata lugensand Nephotettix cinciteps.Entomol Exp Appl 66: 119–126 (1993).
Powell KS, Gatehouse AMR, Hilder VA, Gatehouse JA: Anti-feedant effect of plant lectins and an enzyme on the adult stage of the rice brown planthopper Nilaparvata lugens.Entomol Exp Appl 75: 51–59 (1995).
Powell KS, Gatehouse AMR, Hilder VA, Van Damme EJM, Peumans WJ, Boonjawat J, Horsham K, Gatehouse JA: Different antimetabolic effects of related lectins towards nymphal stages of the rice brown planthopper Nilaparvata lugens.Entomol Exp Appl 75: 61–65 (1995).
Rahbé Y, Sauvion N, Febvay G, Peumans WJ, Gatehouse AMR: Toxicity of lectins and processing of ingested proteins in the pea aphid Acyrthosiphon pisum.Entomol Exp Appl 76: 143–155 (1995).
Sauvion N, Rahbe Y, Peumans WJ, Van Damme EJM, Gatehouse JA, Gatehouse AMR: Effects of GNA and other mannose binding lectins on development and fecundity of the peach-potato aphid Myzus persicae.Entomol Exp Appl 79: 285–293 (1996).
Shade RE, Schroeder HE, Pueyo JJ, Tabe LM, Murdoch, LL, Higgins TJV, Chrispeels MJ: Transgenic peas expressing the α-amylase inhibitor of the common bean are resistant to bruchid beetles. Bio/technology 12: 793–796 (1994).
Shukle RH, Murdoch LL: Lipoxygenase, trypsin inhibitor and lectin from soybeans: effects on larval growth of Manduca sexta.Envir Entomol 12: 787–791 (1983).
Tabashnik BE, Cushing NL, Finson N, Johnson MW: Field development of resistance to Bacillus thuringiensisin diamondback moth (Lepidoptera: Plutellidae). J Econ Entomol 83: 1671–1676 (1990).
Van Damme EJM: Analysis of lectin cDNA clones from Amaryllidaceae and Alliaceae species. Ph. D. thesis, Katholieke Universiteit Leuven (1991).
Van Damme EJM, Allen AK, Peumans, WJ: Isolation and characterisation of a lectin with exclusive specificity towards mannose from snowdrop (Galanthus nivalis) bulbs. FEBS Lett 215: 140–144 (1987).
Van Damme EJM, de Clercq N, Claessens F, Hemschoote K, Peeters B, Peumans, WJ: Molecular cloning and characterisation of multiple isoforms of the snowdrop (Galanthus nivalis) lectin. Planta 186: 35–43 (1991).
Vaeck M, Reynaerts A, Hofte H, Jansens S, De Beuckeleer M, Dean C, Zabeau M, Van Montagu M, Leemans J: Transgenic plants protected from insect attack. Nature 327: 33–37 (1987).
Verwoerd TC, Dekker BMM, Hoekema A: A small-scale procedure for the rapid isolation of plant RNAs. Nucl Acids Res 17: 2362 (1989).
Wenjun S, Forde BG: Efficient transformation of Agrobacteriumspp. by high voltage electroporation. Nucl Acids Res 17: 8385 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gatehouse, A.M., Davison, G.M., Newell, C.A. et al. Transgenic potato plants with enhanced resistance to the tomato moth, Lacanobia oleracea: growth room trials. Molecular Breeding 3, 49–63 (1997). https://doi.org/10.1023/A:1009600321838
Issue Date:
DOI: https://doi.org/10.1023/A:1009600321838