Abstract
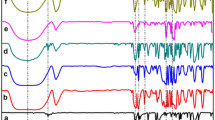
The stability of β-cyclodextrin·ethyl benzoate·6H2O(β-CD·C6H5COOC2H5·6H2O) was investigated by TG and DSC. The mass loss takes place in three stages: the dehydration occurs at 50-120°C; the dissociation of β-CD·C6H5COOC2H5occurs at 200-260°C; the decomposition of β-CD begins at 280°C. The kinetics of the dissociation of β-CD·C6H5COOC2H5in a dry nitrogen flow was studied by means of thermogravimetry both at constant temperature and linearly increasing temperature. The results show that the dissociation of β-CD·C6H5COOC2H5is dominated by a three-dimensional diffusion process (D3). The activation energy E is 116.19 kJ mol-1and the pre-exponential factor A 6.5358·109min-1.
Cyclodextrin is able to form inclusion complexes with a great variety of guest molecules, and the studies focus on the energy of binding between cyclodextrin and the guest molecule. In this paper, the β-cyclodextrin·ethyl benzoate inclusion complex was studied by fluorescence spectrophotometry and infrared absorption spectroscopy, and the results show that the stable energy of inclusion complexes of β-CD with weakly polar guest molecules consists mainly of van der Waals interaction.
Similar content being viewed by others
References
L. H. Tong, Huaxue Tongbao, 1981, (2) 68.
C. Betzel, W. Saenger, B. E. Hingerty and G. M. Brown, J. Am. Chem. Soc., 106 (1984) 7545.
J. Szejtli, Cyclodextrins and their Inclusion Complexes, Akadémiai Kiadó, Budapest, 1982.
C. P. Zhang and Y. G. Zhang, Yaoxue Tongbao, 22 (1987) 101.
J. Olah, Wat. Res., 22 (1988) 1345.
PCT Int. Appl. WO, 91/18525.
J. H. Li and G. E. Zhang, Wulihuaxue Xuebao, 8 (1992) 123.
J. J. Pysiak and B. Sabalski, J. Thermal Anal., 17 (1979) 287.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhang, G.E., Li, X.T., Tian, S.J. et al. Kinetic Studies on the Thermal Dissociation of β-Cyclodextrin·Ethyl Benzoate Inclusion Complexes. Journal of Thermal Analysis and Calorimetry 54, 947–956 (1998). https://doi.org/10.1023/A:1010129028436
Issue Date:
DOI: https://doi.org/10.1023/A:1010129028436