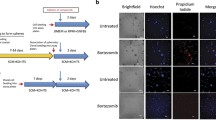
Shortly after reaching confluency, canine MDCK cells enter a prolonged state of basal growth with doubling times of 200–300 hours. These values are similar to those commonly exhibited by in vivo solid tumors at clinically relevant sizes. By comparison with rapidly growing sparse density cultures, the postconfluent monolayers displayed a pronounced resistance to deazauridine, deoxyspergualin, and 5-fluorouridine. Drug concentrations required for unit levels of effect increased from several fold to several orders of magnitude as cells entered high density basal growth. This high density chemoresistance was observed for both growth inhibition and cytotoxicity, but was much more pronounced with the former. Dose-response curves were biphasic, suggesting that growth inhibition and cytotoxicity may be mediated by different mechanisms of drug action. The pronounced chemoresistance of postconfluent MDCK monolayers is similar to that encountered with many clinical solid neoplasms. It suggests that postconfluency monolayers, like multicellular spheroids and cellular multilayers, may provide better in vitro models of solid tumor chemosensitivity than subconfluent monolayer and suspension cultures.
Similar content being viewed by others
Abbreviations
- DAUR:
-
3-deazauridine
- DSG:
-
deoxyspergualin
- 5-FUrd:
-
5-fluorouridine
- HDCR:
-
high-density chemoresistance
- IC50:
-
concentration producing 50% inhibition of growth
- LC50:
-
concentration producing 50% loss of cell protein
References
BACHUR, N.R., GORDON, S.L. and GEE, M.V. (1978). A general mechanism for microsomal activation of quinone anticancer agents to free radicals. Cancer Res. 38:1745–1750.
BARRANCO, S.C. (1984). Cellular and molecular effects of adriamycin on dividing and nondividing cells. Pharmacol. Ther. 24:303–319.
BICHAY, T.J. and INCH, W.R. (1985). The in vitro cytoxicity of mitoxantrone to V79 cells grown as monolayers and as multicell spheroids. Proc. Am. Assoc. Cancer Res. 26:354.
CHAMBERS, S.H., BLEEHAN, N.M. and WATSON, J.V. (1984). Effect of cell density on intracellular adriamycin concentration and cytotoxicity in exponential and plateau phase EMT6 cells. Br. J. Cancer 49:301–306.
DEVITA, V.T. and SCHEIN, P.S. (1983). The use of drugs in combination for the treatment of cancer. N. Engl. Med. 288:998–1006.
GRAY, J.W. (1983). Quantitative cytokinetics: cellular response to cell cycle specific agents. Pharmacol. Ther. 22: 163–197.
HAGEMANN, R.F., SCHENKEN, L.L. and LESHER, S. (1973). Tumor chemotherapy: efficiency dependent on mode of growth. J. Natl. Cancer Inst. 50:467–474.
JACKSON, R.C. (1984). Biological effects of folic acid antagonists with antineoplastic activity. Pharmacol. Ther. 25:61–82.
KRUSE, P.F. and MIEDEMA, E. (1965). Production and characterization of multiple-layered perfusion cultures. J. Natl. Cancer Inst. 31:273–279.
KWOK, T.T. and TWENTYMAN, P.R. (1985). The response to cytotoxic drugs of EMT6 cells treated either as intact or disaggregated spheroids. Br. J. Cancer 51:211–218.
MARSONI, S. and WITTES, R. (1984). Clinical development of anticancer agents—a National Cancer Institute perspective. Cancer Treat. Rep. 68:77–85.
MOMPARLER, R.L., KARON, M., SIEGEL, S.E. and AVILA, F. (1976). Effect of adriamycin on DNA, RNA, and protein synthesis in cell-free systems and intact cells. Cancer Res. 36:2891–2895.
NARAYANAN, V.L. (1986). New anticancer leads at the National Cancer Institute and new approaches to their discovery. Bristol-Myers Cancer Research Symposium 8, in press.
NEDERMAN, T. (1984). Effect of vinlastine and 5-fluorouracil on human glioma and thyroid cancer monolayers and spheroids. Cancer Res. 44:254–258.
OVAJERA, A.A. and HOUCHENS, D.P. (1981). Human tumor xenografts in athymic nude mice as a preclinical screen for anticancer agents. Semin. Oncol. 8:386–393.
SALMON, S.E. (1984). Human tumor colony assay and chemosensitivity testing. Cancer Treat. Rep. 68:117–125.
SKEHAN, P. (1976). On the regulation of cell growth. Exp. Cell Res. 97:184–192.
SKEHAN, P. (1984). Cell growth, tissue, neogenesis, and neoplastic transformation. In: Growth, Cancer, and the Cell Cycle (P. Skehan and S.J. Friedman, eds.), pp. 323–345. Human Press, New York.
SKEHAN, P. and FRIEDMAN, S.J. (1985a). A rapid naphthol yellow S method for measuring the cell protein content of anchorage cultures. In Vitro 21:288–290.
SKEHAN, P. and FRIEDMAN, S.J. (1985b). Objective growth criteria for chemosensitivity studies in preclinical tumor models. Proc. Am. Assoc. Cancer Res. 26:333
SKIPPER, H.E. and SCHABEL, F.M. (1984). Tumor stem cell heterogeneity: implications with respect to classification of cancers by chemotherapeutic effect. Cancer Treat. Rep. 68:43–61.
STAQUET, M.J., BYAR, D.P., GREEN, S.B., and ROZENCWEIG, M. (1983). Clinical predictivity of transplantable tumor systems in the selection of new drugs for solid tumors: rationale for a three-stage strategy. Cancer Treat. Rep. 67:753–765.
STEEL, G.G. (1977). Growth Kinetics of Tumors. Clarendon Press, Oxford.
SUTHERLAND, R.M., EDDY, H.A., BAUHAM, B., REICH, K. and VANAUTWERP, D. (1979). Resistance to adriamycin in multicellular spheroids. Int. J. Radiat. Oncol. Biol. Phys. 5:1225–1230.
TRAGONOS, F. (1983). Dihydroxyanthraquinone and related Bis(substituted) aminoanthraquinones: a novel class of antitumor agents. Pharmacol. Ther. 22:194–214.
TWENTYMAN, P.R. (1984). Bleomycin—mode of action with particular reference to the cell cycle. Pharmacol. Ther. 23:417–441.
VALERIOTE, F.A. and SANTELLI, G. (1994). 5-Fluorouracil (FUra) Pharmacol. Ther. 24:107–132.
VENDITTI, J.M. (1981). Preclinical drug development: rationale and methods. Semin. Oncol. 8:349–361.
WIBE, E. and OFTEBRO, R. (1981). A study of factors related to the action of 1-propargyl-5-chloropyrimidine-2-one (NY-3170) and vincristine in human multicellular spheroids. Int. J. Cancer Clin. Oncol. 17: 1053–1059.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Skehan, P., Thomas, J. & Friedman, S.J. Postconfluency MDCK monolayers as an in vitro model of solid tumor chemosensitivity. Cell Biol Toxicol 2, 357–368 (1986). https://doi.org/10.1007/BF00121851
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00121851