Abstract
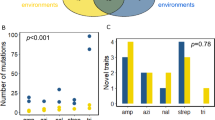
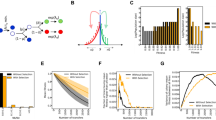
An important problem in microbial ecology is to identify those phenotypic attributes that are responsible for competitive fitness in a particular environment. Thousands of papers have been published on the physiology, biochemistry, and molecular genetics of Escherichia coli and other bacterial models. Nonetheless, little is known about what makes one genotype a better competitor than another even in such well studied systems. Here, we review experiments to identify the phenotypic bases of improved competitive fitness in twelve E. coli populations that evolved for thousands of generations in a defined environment, in which glucose was the limiting substrate. After 10000 generations, the average fitness of the derived genotypes had increased by ∼ 50% relative to the ancestor, based on competition experiments using marked strains in the same environment. The growth kinetics of the ancestral and derived genotypes showed that the latter have a shorter lag phase upon transfer into fresh medium and a higher maximum growth rate. Competition experiments were also performed in environments where other substrates were substituted for glucose. The derived genotypes are generally more fit in competition for those substrates that use the same mechanism of transport as glucose, which suggests that enhanced transport was an important target of natural selection in the evolutionary environment. All of the derived genotypes produce much larger cells than does the ancestor, even when both types are forced to grow at the same rate. Some, but not all, of the derived genotypes also have greatly elevated mutation rates. Efforts are now underway to identify the genetic changes that underlie those phenotypic changes, especially substrate specificity and elevated mutation rate, for which there are good candidate loci. Identification and subsequent manipulation of these genes may provide new insights into the reproducibility of adaptive evolution, the importance of co-adapted gene complexes, and the extent to which distinct phenotypes (e.g., substrate specificity and cell size) are affected by the same mutations.
Similar content being viewed by others
References
Akerlund T, Nordström K & Bernander R (1995) Analysis of cell size and DNA content in exponentially growing and stationary-phase batch cultures of Escherichia coli. Journal of Bacteriology 177: 6791-6797
Bremer H & Dennis PP (1987) Modulation of chemical composition and other parameters of the cell by growth rate. In: Neidhardt FC, Ingraham JL, Low KB, Magasanik B, Schaechter M & Umbarger HE (Eds) Escherichia coliand Salmonella typhimurium: Cellular and Molecular Biology, Vol 2 (pp 1527-1542). American Society for Microbiology, Washington, DC
Chao L & Cox EC (1983) Competition between high and low mutating strains of Escherichia coli. Evolution 37: 125-134
Cox EC (1976) Bacterial mutator genes and the control of spontaneous mutation. Annual Review of Genetics 10: 135-156
Drake JW (1991) A constant rate of spontaneous mutation in DNA-based microbes. Proceedings of the National Academy of Sciences USA 88: 7160-7164
Dykhuizen DE & Dean AM (1990) Enzyme activity and fitness: evolution in solution. Trends in Ecology & Evolution 5: 257-262
Dykhuizen DE & Hartl DL (1983) Selection in chemostats. Microbiological Reviews 47: 150-168
Elena SF, Cooper VS & Lenski RE (1996) Punctuated evolution caused by selection of rare beneficial mutations. Science 272: 1802-1804
Gerrish PJ & Lenski RE (1998) The fate of competing beneficial mutations in an asexual population. Genetica: in press
Hall BG (1983) Evolution of new metabolic functions in laboratory organisms. In: Nei M & Koehn RK (Eds) Evolution of Genes and Proteins (pp 234-257). Sinauer Associates, Sunderland, Massachusetts, USA
Horiuchi T, Maki H & Sekiguchi M (1989) Mutators and fidelity of DNA replication. Bulletin Institut Pasteur 87: 309-336
Kimura M (1983) The Neutral Theory of Molecular Evolution. Cambridge University Press, Cambridge
Lenski RE (1992) Experimental evolution. In Lederberg J (Ed) Encyclopedia of Microbiology, Vol 2 (pp 125-140). Academic Press, San Diego
Lenski RE (1995) Molecules are more than markers: new directions in molecular microbial ecology. Molecular Ecology 4: 643-651
Lenski RE & Travisano M (1994) Dynamics of adaptation and diversification: A 10,000generation experiment with bacterial populations. Proceedings of the National Academy of Sciences, USA 91: 6808-6814
Lenski RE, Rose MR, Simpson SC & Tadler SC (1991) Longterm experimental evolution in Escherichia coli. I. Adaptation and divergence during 2,000 generations. The American Naturalist 138: 1315-1341
Levin BR & Lenski RE (1983) Coevolution in bacteria and their viruses and plasmids. In: Futuyma DJ & Slatkin M (Eds) Coevolution (pp 99-127). Sinauer Associates, Sunderland, Massachusetts, USA
Luria SE & Delbrück M (1943) Mutations of bacteria from virus sensitivity to virus resistance. Genetics 28: 491-511
Mongold JA & Lenski RE (1996) Experimental rejection of a nonadaptive explanation for increased cell size in Escherichia coli. Journal of Bacteriology 178: 5333-5334
Mortlock RP, Ed (1984) Microorganisms as Model Systems for Studying Evolution. Plenum Press, New York
Neidhardt FC, Curtiss R, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M & Umbarger HE, Eds (1996) Escherichia coliand Salmonella typhimurium: Cellular and Molecular Biology, 2nd Edition. American Society for Microbiology, Washington, DC
Price TD, Grant PR, Gibbs HL & Boag PT (1984) Recurrent patterns of natural selection in a population of Darwin's finches. Nature 309: 787-789
Schaechter M, Maaløe O & Kjelgaard NO (1958) Dependence on medium and temperature of cell size and chemical composition during balanced growth of Salmonella typhimurium. Journal of General Microbiology 19: 592-606
Schmidt TM (1996) Multiplicity of ribosomal operons in prokaryotic genomes. In: de Bruijn FJ, Lupski JR & Weinstock G (Eds) Bacterial Genomes: Physical Structure and Analysis. Chapman and Hall, New York
Sniegowski PD, Gerrish PJ & Lenski RE (1997) Evolution of high mutation rates in experimental populations of Escherichia coli. Nature 387: 703-705
Taddei F, Radman M, Maynard Smith J, Toupance B, Gouyon PH & Godelle B (1997) Role of mutator alleles in adaptive evolution. Nature 387: 700-702
Travisano M & Lenski RE (1996) Long-term experimental evolution in Escherichia coli. IV. Targets of selection and the specificity of adaptation. Genetics 143: 15-26
Travisano M, Vasi F & Lenski RE (1995) Long-term experimental evolution in Escherichia coli. III. Variation among replicate populations in correlated responses to novel environments. Evolution 49: 189-200
Vasi F, Travisano M & Lenski RE (1994) Long-term experimental evolution in Escherichia coli. II. Changes in life-history traits during adaptation to a seasonal environment. The American Naturalist 144: 432-456
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lenski, R.E., Mongold, J.A., Sniegowski, P.D. et al. Evolution of competitive fitness in experimental populations of E. coli: What makes one genotype a better competitor than another?. Antonie Van Leeuwenhoek 73, 35–47 (1998). https://doi.org/10.1023/A:1000675521611
Issue Date:
DOI: https://doi.org/10.1023/A:1000675521611