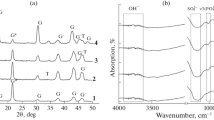
Two examples of zinc polycarboxylate dental cement were studied, one of which was prepared from an aqueous solution of poly (acrylic acid) together with the zinc oxide powder, the other being prepared by adding water to a mixture of dried polyacid and zinc oxide powder. The changes in the properties of the resultant cements with length of storage in various media were determined. In all cases the maximum strength was achieved fairly rapidly, usually at 1 week, after which there was little or no increase. Cements stored in water achieved the lowest compressive strengths, whereas cements stored in highly desiccating conditions, over concentrated sulphuric acid, achieved very high (if variable) compressive strengths. There appeared to be very little difference between the water-activated and conventional cements. These results confirm previous findings that zinc polycarboxylate cements are relatively poorly hydrated compared with other polyelectrolyte biomaterials. This in turn implies that water does not play a structural role in these cements.
Similar content being viewed by others
References
D. C. SMITH. Brit. Dent. J. 125 (1968) 381.
D. F. WILLIAMS and J. CUNNINGHAM. “Materials in clinical dentistry” (Oxford University Press, Oxford, 1979).
A. D. WILSON. Chem. Soc. Rev. 7 (1978) 254.
N. N. GREENWOOD and A. EARNSHAW, “The chemistry of the elements” (Pergamon Press, Oxford, 1984).
A. D. WILSON, J. Biomed. Mater. Res. 16 (1982) 549.
J. W. NICHOLSON, O. M. LACY, G. S. SAYERS and A. D. WILSON, J. Biomed. Mater. Res. 22 (1988) 623.
M. A. MOHARAN, H. ABDEL-HAKEEN, H. SHAHEEN and R. M. IBRAHIM, J. Mater. Sci. 21 (1986) 1681.
S. M. RABIE, A. SAWARBY, M. A. MOHARAN, A. M. NASSER and K. H. TAHAN, J. Appl. Polym. Sci. 41 (1990) 445.
R. C. MEHROTRA and R. BOHRA. “Metal carboxylates” (Academic Press, London, 1983).
A. D. WILSON, in Scientific aspects of dental materials”, edited by J. A. Fraunhofer (Butterworths, London, 1975).
S. CRISP, B. G. LEWIS and A. D. WILSON, J. Dent. Res. 55 (1976) 299.
W. D. COOK, Biomaterials 3 (1982) 232.
R. G. HILL and S. A. LABOK, J. Mater. Sci. 26 (1991) 67.
J. M. PADDON and A. D. WILSON, J. Dent. 4 (1976) 183.
A. D. WILSON and J. W. NICHOLSON, “Special cements: acid-base reaction cements; their dental and other applications” (Cambridge University Press) in press.
A. D. WILSON, J. M. PADDON and S. CRISP, J. Dent. Res. 58 (1979) 1065.
A. D. WILSON and J. W. McLEAN, “Glass-ionomer cement” (Quintessence Books, Chicago, 1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nicholson, J.W., Hawkins, S.J. & Wasson, E.A. A study of the structure of zinc polycarboxylate dental cements. J Mater Sci: Mater Med 4, 32–35 (1993). https://doi.org/10.1007/BF00122974
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00122974