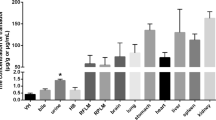
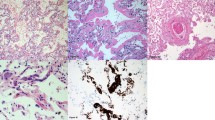
Abstract
IN 1928 Corson and Stoughton1 prepared a number of benzylidene malononitriles, including 2-chlorobenzylidene malononitrile (CS). Although these authors were primarily concerned with the isolation and chemistry of the condensation products of malononitrile and various aldehydes, sternutatory and irritant properties on the part of some of the compounds were reported. CS is now the active material in a wide range of devices used against civil disturbances and in war (see, for example, refs. 2–6). Benzylidene malononitriles are reactive substances, and have been shown to form adducts with amines7–10, n-butanethiol10–12, Grignard reagents13,14, hydrazoic acid15, hydrogen cyanide1,15–17, salts of certain transition elements18,19, and trialkylphosphines20–22. Reactions also occur with hypochlorite23–24, water1,9,17,25,26, oxidizing1 and reducing7,27,28 agents. Certain aspects of the physiological effects2,7,8,29–32 and toxic properties23,33–38 of CS have also been studied. We here describe a brief investigation into the mechanism of toxicity of CS. Because elevated levels of thiocyanate have been found in the blood of human patients treated with 4-nitrobenzylidene malononitrile39, and because malononitrile is one of the products when CS is hydrolysed in aqueous ethanol9, the toxicities of cyanide and malononitrile have also received attention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Corson, B. B., and Stoughton, R. W., J. Amer. Chem. Soc., 50, 2835 (1928).
Finn, D. H., Hogg, M. A. P., and Crichton, D., Brit. Patent, 967, 660 (1964).
White, G. T. and Rothstein, L. R., US Patent, 3,314,835 (1967).
Bryant, P. J. R., Owen, A. R., and Scanes, F. S., US Patent, 3,391,036 (1968).
Ministry of Technology, Brit. Patent, 1,139,761 (1969).
Bryant, P. J. R., Owen, A. R., and Scanes, F. S., Brit. Patent, 1,164,991 (1969).
Holmes, H. L., Suffield Technical Paper No. 231 (1962).
Holmes, H. L., Suffield Technical Paper No. 234 (1962).
Patai, S., and Rappoport, Z., J. Chem. Soc., 383 (1962).
Silver, R. F., Kerr, K. A., Frandsen, P. A., Kelley, S. J., and Holmes, H. L., Canad. J. Chem., 45, 1001 (1967).
Lough, C. E., Currie, D. J., and Holmes, H. L., Canad. J. Chem., 46, 772 (1968).
Pritchard, R. B., Lough, C. E., Currie, D. J., and Holmes, H. L., Canad. J. Chem., 46, 775 (1968).
Gupte, S. D., and Sunthankar, S. V., J. Org. Chem., 24, 1334 (1959).
Newman, M. S., and Phillips, D. K., J. Amer. Chem. Soc., 81, 3667 (1959).
Ostroverkhov, V. G., and Shilov, E. A., Ukr. Khim. Zh., 27, 209 (1961); Chem. Abstr., 55, 23302a (1961).
Almange, J. P., and Carrie, R., CR Hebd. Séanc. Acad. Sci., 257, 1781 (1963).
Pritchard, R. B., Lough, C. E., Reesor, J. B., Holmes, H. L., and Carrie, D. J., Canad. J. Chem., 45, 775 (1967).
Schrauzer, G. N., and Eichler, S., Chem. Ber., 95, 260 (1962).
Schrauzer, G. N., Eichler, S., and Brown, D. A., Chem. Ber., 95, 2755 (1962).
Horner, L., and Klüpfel, K., Justus Liebigs Ann. Chem., 591, 69 (1955).
Horner, L., German Patent, 937,587 (1956).
Rappoport, Z., and Gertler, S., J. Chem. Soc., 1360 (1964).
Punte, C. L., Owens, E. J., and Gutentag, P. J., Archs. Envir. Hlth., 6, 366 (1963).
Rosenblatt, D. H., and Broome, G. H., J. Org. Chem., 28, 1290 (1963).
Patai, S., and Rappoport, Z., J. Chem. Soc., 392 (1962).
Campaigne, E., Bulbenko, G. F., Kreighbaum, W. E., and Maulding, D. R., J. Org. Chem., 27, 4428 (1962).
Bargain, M., CR Hebd. Séanc. Acad. Sci., 254, 130 (1962).
Bargain, M., CR Hebd. Séanc. Acad. Sci., 255, 1948 (1962).
Biscoe, T. J., and Shephard, R. J., Archs. Intern. Pharmacodyn. Thér., 138, 389 (1963).
Owens, E. J., and Punte, C. L., Amer. Ind. Hyg. Ass. J., 24, 262 (1963).
Hellreich, A., Goldman, R. H., Bottiglieri, N. G., and Weimer, J. T., Edgewood Arsenal Technical Rep., 4075 (1967).
Rengstorff, R. H., Milit. Med., 134, 219 (1969).
Crichton, D., Porton Technical Paper, 672 (1959).
Bowers, M. B., Owens, E. J., and Punte, C. L., CWL Technical Memo, 24–50 (1960).
Punte, C. L., Weimer, J. T., Ballard, T. A., and Wilding, J. L., Toxic. Appl. Pharmac., 4, 656 (1962).
Directorate of Medical Research, Special Summary Report (Edgewond Arsenal, Maryland, 1965).
Striker, G. E., Streett, C. S., Ford, D. F., Herman, L. H., and Helland, D. R., Edgewood Arsenal Technical Rep., 4071 (1967).
Striker, G. E., Streett, C. S., Ford, D. F., Herman, L. H., and Helland, D. R., Edgewood Arsenal Technical Rep., 4072 (1967).
Petrakis, N. L., Bierman, H. R., and Shimkin, M. B., Cancer Res., 12, 573 (1952).
Litchfield, J. T., and Fertig, J. W., Bull. Johns Hopkins Hosp., 69, 276 (1941).
Miller, L. C., and Tainter, M. L., Proc. Soc. Exp. Biol. Med., 57, 261 (1944).
Nash, J. B., Doucet, B. M., Ewing, P. L., and Emerson, G. A., Archs. Intern. Pharmacodyn. Thér., 84, 385 (1950).
Heymans, J. F., and Masoin, P., Archs. Intern. Pharmacodyn. Thér., 3, 77 (1897).
Stern, J., Weil-Malherbe, H., and Green, R. H., Biochem. J., 52, 114 (1952).
Lang, S., Arch. Exp. Path. Pharmak., 34, 247 (1894).
Bovell, C. R., Packer, L., and Schonbaum, G. R., Arch. Biochem. Biophys., 104, 458 (1964).
Pearson, R. G., and Dillon, R. L., J. Amer. Chem. Soc., 75, 2439 (1953).
Panov, I., Khig. Zdraveopazvane, 9, 50 (1966); Chem. Abstr., 65, 11221d (1966).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
JONES, G., ISRAEL, M. Mechanism of Toxicity of Injected CS Gas. Nature 228, 1315–1317 (1970). https://doi.org/10.1038/2281315a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/2281315a0
This article is cited by
-
Cyanide and fire victims: A hazard in focus
Fire Technology (1990)
-
Formation of cyanide from o-chlorobenzylidene malononitrile and its toxicological significance
Archiv f�r Toxikologie (1973)
-
CS and its Chemical Relatives
Nature (1972)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.