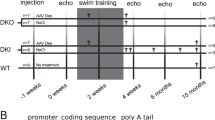
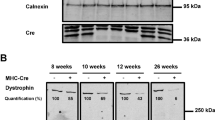
Abstract
Both enteroviral infection of the heart and mutations in the dystrophin gene can cause cardiomyopathy. Little is known, however, about the interaction between genetic and acquired forms of cardiomyopathy. We previously demonstrated that the enteroviral protease 2A cleaves dystrophin; therefore, we hypothesized that dystrophin deficiency would predispose to enterovirus-induced cardiomyopathy. We observed more severe cardiomyopathy, worsening over time, and greater viral replication in dystrophin-deficient mice infected with enterovirus than in infected wild-type mice. This difference appears to be a result of more efficient release of the virus from dystrophin-deficient myocytes. In addition, we found that expression of wild-type dystrophin in cultured cells decreased the cytopathic effect of enteroviral infection and the release of virus from the cell. We also found that expression of a cleavage-resistant mutant dystrophin further inhibited the virally mediated cytopathic effect and viral release. These results indicate that viral infection can influence the severity and penetrance of the cardiomyopathy that occurs in the hearts of dystrophin-deficient individuals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Seidman, J.G. & Seidman, C. The genetic basis for cardiomyopathy: From mutation identification to mechanistic paradigms. Cell 104, 557–567 (2001).
Feldman, A.M. & McNamara, D. Myocarditis N. Engl. J. Med. 343, 1388–1398 (2000).
Towbin, J.A., Bowles, K.R. & Bowles, N.E. Etiologies of cardiomyopathy and heart failure. Nature Med. 5, 266–267 (1999).
Martino, T.A., Liu, P. & Sole, M.J. Viral infection and the pathogenesis of dilated cardiomyopathy. Circ. Res. 74, 182–188 (1994).
Baboonian, C., Davies, M.J., Booth, J.C. & McKenna, W.J. Coxsackie B viruses and human heart disease. Curr. Top. Microbiol. Immunol. 223, 31–52 (1997).
Koenig, M., Monaco, A.P. & Kunkel, L.M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell 53, 219–226 (1988).
Goldberg, S.J. et al. Serial left ventricular wall measurements in Duchenne's muscular dystrophy. J. Am. Coll. Cardiol. 2, 136–142 (1983).
Danilowicz, D., Rutkowski, M., Myung, D. & Schively, D. Echocardiography in duchenne muscular dystrophy. Muscle Nerve 3, 298–303 (1980).
Nigro, G., Comi, L.I., Politano, L. & Bain, R.J. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int. J. Cardiol. 26, 271–277 (1990).
Towbin, J.A. et al. X-linked dilated cardiomyopathy. Molecular genetic evidence of linkage to the Duchenne muscular dystrophy dystrophin gene at the Xp21 locus. Circulation 87, 1854–1865 (1993).
Muntoni, F. et al. Brief report: Deletion of the dystrophin muscle-promoter region associated with X-linked dilated cardiomyopathy. N. Engl. J. Med. 329, 921–925 (1993).
Ortiz-Lopez, R., Li, H., Su, J., Goytia, V. & Towbin, J.A. Evidence for a dystrophin missense mutation as a cause of X-linked dilated cardiomyopathy. Circulation 95, 2434–2440 (1997).
Badorff, C. et al. Enteroviral protease 2A cleaves dystrophin: Evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nature Med. 5, 320–326 (1999).
Badorff, C., Berkley, N., Mehrotra, S., Rhoads, R.E. & Knowlton, K.U. Enteroviral protease 2A directly cleaves dystrophin and is inhibited by a dystrophin-based substrate analogue. J. Biol. Chem. 275, 1191–1197 (2000).
Lee, G.H., Badorff, C. & Knowlton, K.U. Dissociation of sarcoglycans and the dystrophin carboxyl terminus from the sarcolemma in enteroviral cardiomyopathy. Circ. Res. 87, 489–495 (2000).
Knowlton, K.U., Jeon, E.S., Berkley, N., Wessely, R. & Huber, S. A mutation in the puff region of VP2 attenuates the myocarditic phenotype of an infectious cDNA of the Woodruff variant of CVB3. J. Virol. 70, 7811–7818 (1996).
Straub, V., Rafael, J.A., Chamberlain, J.S. & Campbell, K.P. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J. Cell Biol. 139, 375–385 (1997).
Cohn, R.D. et al. Prevention of cardiomyopathy in mouse models lacking the smooth muscle sarcoglycan-sarcospan complex. J. Clin. Invest. 107, R1–R7 (2001).
Bergelson, J.M. et al. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J. Virol. 72, 415–419 (1998).
Clemens, P.R. et al. Recombinant truncated dystrophin minigenes: Construction, expression, and adenoviral delivery. Hum. Gene Ther. 6, 1477–1485 (1995).
Lee, C.L., Pearlman, J.A., Chamberlain, J.S. & Caskey, C.T. Expression of recombinant dystrophin and its localization to the cell membrane. Nature 349, 334–336 (1991).
Petrof, B.J., Shrager, J.B., Stedman, H.H., Kelly, A.M. & Sweeney, H.L. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc. Natl. Acad. Sci. USA 90, 3710–3714 (1993).
Clarke, M.S., Khakee, R. & McNeil, P.L. Loss of cytoplasmic basic fibroblast growth factor from physiologically wounded myofibers of normal and dystrophic muscle. J. Cell. Sci. 106 (Pt 1), 121–133 (1993).
Ervasti, J.M. & Campbell, K.P. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J. Cell Biol. 122, 809–823 (1993).
Rafael, J.A. et al. Forced expression of dystrophin deletion constructs reveals structure-function correlations. J. Cell Biol. 134, 93–102 (1996).
Rueckert, R.R. Fundamental Virology. (eds. Fields, B.N., Knipe, D.M. & Howley, P.M.) 477–522 (Raven, New York, 1996).
Luftig, R.B. & Lupo, L.D. Viral interactions with the host-cell cytoskeleton: The role of retroviral proteases. Trends Microbiol. 2, 178–182 (1994).
Jackson, P. & Bellett, A.J. Reduced microfilament organization in adenovirus type 5-infected rat embryo cells: a function of early region 1a. J. Virol. 55, 644–650 (1985).
Zhang, Y. & Schneider, R.J. Adenovirus inhibition of cell translation facilitates release of virus particles and enhances degradation of the cytokeratin network. J. Virol. 68, 2544–2555 (1994).
Shoeman, R.L. et al. Human immunodeficiency virus type 1 protease cleaves the intermediate filament proteins vimentin, desmin, and glial fibrillary acidic protein. Proc. Natl. Acad. Sci. USA 87, 6336–6340 (1990).
Seipelt, J., Liebig, H.D., Sommergruber, W., Gerner, C. & Kuechler, E. 2A proteinase of human rhinovirus cleaves cytokeratin 8 in infected HeLa cells. J. Biol. Chem. 275, 20084–20089 (2000).
Vatta, M. et al. Molecular remodelling of dystrophin in patients with end-stage cardiomyopathies and reversal in patients on assistance-device therapy. Lancet 359, 936–941 (2002).
Henke, A., Huber, S., Stelzner, A. & Whitton, J.L. The role of CD8+ T lymphocytes in coxsackievirus B3-induced myocarditis. J. Virol. 69, 6720–6728 (1995).
Acknowledgements
We thank R. Myers and D. Rappaport for providing equipment and advice for cryosections, immunostaining and imaging; J. Chen and S. Evans for helpful discussions; A. Henke for the anti-CVB3 antibody; and P.R. Clemens and C.C. Lee for providing the mouse dystrophin expression vector. This work was supported in part by a US National Institutes of Health grant (5 R01 HL57365-03) and an American Heart Association Established Investigator Award (to K.U.K.); by Our Lady of Mercy Hospital, Catholic University of Korea (to G.H.L.); and by a training grant from the Deutsche Forschungsgemeinschaft (Ba 1668/1-1) (to C.B.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Xiong, D., Lee, GH., Badorff, C. et al. Dystrophin deficiency markedly increases enterovirus-induced cardiomyopathy: A genetic predisposition to viral heart disease. Nat Med 8, 872–877 (2002). https://doi.org/10.1038/nm737
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm737
This article is cited by
-
Myocarditis in Athletes: Risk Factors and Relationship with Strenuous Exercise
Sports Medicine (2024)
-
Myocarditis: Which Role for Genetics?
Current Cardiology Reports (2021)
-
Severe cardiac involvement with preserved truncated dystrophin expression in Becker muscular dystrophy by +1G>A DMD splice-site mutation: a case report
Journal of Human Genetics (2020)
-
Specific elimination of coxsackievirus B3 infected cells with a protein engineered toxin-antitoxin system
Molecular & Cellular Toxicology (2019)
-
Adiponectin promotes coxsackievirus B3 myocarditis by suppression of acute anti-viral immune responses
Basic Research in Cardiology (2014)