Abstract
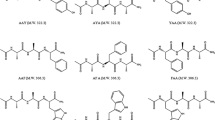
Two series of sequential poly(Lys-X) (X: Gly, βAla, and ɛAhx in series A; Gly, Ala, Leu and Phe in series B) have been synthesized. On the chiral interaction between cationic polypeptides and methyl orange (MO), the effects of the distance (series A) and of the hydrophobic side chains (series B) were examined by means of the absorption and induced circular dichroism (CD) spectroscopies. Dichroic bands associated with the blue shifted absorption peaks or shoulders of MO in the visible wavelength region were observed by complex formation between the polypeptides and the dye. The intensity of the induced CD was affected by the concentration of the complexes and time after preparing the complex solutions, suggesting the formation of the intermolecular aggregation in some instances. When MO molecules bound to lysine residues are apart from each other, the aggregation of the complexes is not marked. Roughly, the intensity of the induced CD decreases with increasing distance between the intramolecular lysine residues in series A polypeptides. When the hydrophobicity of the side chains is increased, the induced CD spectra of the series B polypeptide-MO complexes exhibits the inversion of the sign of the induced CD extrema.
Similar content being viewed by others
References
IUPAC-IUB nomenclature (1972) Biochem 11:1726; Biopolym 11:321; ɛ-aminohexanoic acid is abbreviated as ɛAhx
Blout ER, Stryer L (1959) Proc Natl Acad Sci US 45:1591
Stryer L, Blout ER (1961) J Amer Chem Soc 83:1411
Ballard RE, McCaffery AJ, Mason SF (1966) Biopolym 4:97
Yamamoto H, Nakazawa A (1984) Biopolym 23:1367
Harada N, Chen SL, Nakanishi K (1975) J Amer Chem Soc 97:5345
Hatano M, Yoneyama M, Sato Y, Kawamura Y (1973) Biopolym 12:2423
Ando Y (1981) PhD Thesis, Tokyo Institute of Technology
Murakami K, Sano T, Kure N, Ishii K, Yasunaga T (1983) Biopolym 22:1935
Yamamoto H, Nakazawa A, Hayakawa T (1983) J Polym Sci, Polym Lett Edn 21:131
Yamamoto H (1983) Makromol Chem 184:1479
Yamamoto H, Nakazawa A, Hayakawa T, Mitsuishi M (1984) Polym J 16:791
Takagishi T, Kuroki N, Shima S, Sakai H, Yamamoto H, Nakazawa A (1985) J Polym Sci, Polym Lett Edn 23:329
Yamamoto H (1986) Int J Biol Macromol 8:130
Yamamoto H, Nakazawa A (1983) Bull Chem Soc Japan 56:2535
Yamamoto H, Nakazawa A, Hayakawa T, Nishi N, Naruse T (1984) Bull Chem Soc Japan 57:1699
Yamamoto H, Nakazawa A, Hayakawa T, Nishi N (1985) Int J Biol Macromol 7:167
Nishi N, Nakajima B, Hasebe N, Noguchi J (1980) Int J Biol Macromol 2:53
Nakajima B, Nishi N (1981) Polym J 13:183
Nakajima B, Hirata K, Nishi N, Noguchi J (1981) Int J Biol Macromol 3:46
Naruse T, Nakajima B, Tsutsumi A, Nishi N (1981) Polym J 13:1151
Nishi N, Nakajima B (1982) Int J Biol Macromol 4:281
Hatano M, Yoneyama M (1970) J Amer Chem Soc 92:1392
Sarker PK, Doty P (1966) Proc Natl Acad Sci US 55:981
Seipke G, Arfmann HA, Wagner KG (1980) Biopolym 19:189
Yamamoto H, Nakazawa A (1984) Polym Preprints Japan 33:344
Kasha M, Rawls HR, El-Bayoumi MA (1965) Pure Appl Chem 11:371
Tinoco Jr I (1962) Advan Chem Phys 4:113
Urry DW (1969) Spectroscopic Approaches to Biomolecular Conformation, American Medical Assoication, p 33
Némethy G (1967) Angewandte Chem 79:260
Ikeda S, Yamaguchi M (1972) Koll Z u Z Polym 250:311
Ikeda S, Imae T (1973) Polym J 4:301
Ikeda S, Yoshida T, Imae T (1981) Biopolym 20:2395
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yamamoto, H., Nishida, A., Hayakawa, T. et al. Chiral interaction of sequential lysine polypeptides with methyl orange. Effects of distance between lysine residues and hydrophobic side chains. Colloid & Polymer Sci 264, 779–785 (1986). https://doi.org/10.1007/BF01500753
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01500753