Abstract
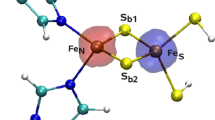
The recombinant high-potential iron-sulfur protein (HiPIP) iso-I from Ectothiorhodospira halophila has been mutated at position 68. The αC of Val 68 is within a 0.6-nm sphere from the closest iron ion of the cluster. The valine residue has been replaced by a negatively charged glutamate residue (V68E) and by a positively charged lysine residue (V68K). With respect to the recombinant wild-type protein the reduction potentials of the V68E and V68K variants are –21±2 and +29±2 mV respectively (200 mM NaCl, pH 7, 25 °C). The solution structure of the V68E mutant was solved up to a pairwise RMSD of 66 pm for backbone atoms and 138 pm for all heavy atoms. The structure of the variant is very similar to that of recombinant wild type, indicating that the observed changes in reduction potentials are largely due to the effect of the introduced charges. It is proposed that the valence distribution within the oxidized iron-sulfur cluster is affected only slightly by the change in charge at position 68, but consistently with a simple electrostatic model.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 2 January 1996 / Accepted: 28 February 1996
Rights and permissions
About this article
Cite this article
Bertini, I., Borsari, M., Bosi, M. et al. The influence of a surface charge on the electronic and steric structure of a high potential iron-sulfur protein. JBIC 1, 257–263 (1996). https://doi.org/10.1007/s007750050051
Issue Date:
DOI: https://doi.org/10.1007/s007750050051