Abstract
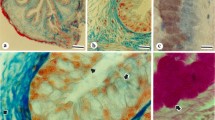
Using an affinity purified antibody raised against the RI-H fragment of rat intestinal lectin L-36, the latter protein has been identified within the esophageal epithelium by means of ultracryotomy followed by immunogold labeling. The epithelium consists of 4 morphologically distinct cell-types, namely, the basal, spiny, granular and squamous cells, and each of these exhibits a different immunolabeling pattern. The basal cells form a layer on the basal lamina, and in these a diffuse cytoplasmic staining is observed. This basal cell layer is overlaid by spiny cells that extend many cell processes into wide intercellular spaces. In these cells, immunogold particles are found only on small granular inclusions consisting of an electron-lucent homogeneous substance. The granular cells from a third layer over the spiny cells, and are characterized by a number of large granular inclusions with an electron-dense core rimmed by a less electron-dense substance. Immunogold labeling is found on these granules, both on the core and peripheral region. Squamous cell-types constitute the most superficial layer of the epithelium. They are without granular inclusions, and immunogold labeling is confined to the cytoplasmic surface of the thickened plasma membrane. These findings suggest that L-36 is produced in the basal cells as free cytosolic protein, then becomes progressively aggregated into the granular inclusions of the spiny and granular cells, and is eventually transferred onto the cytoplasmic surface of the squamous cell plasma membrane where it may interact with complementary glycoconjugate(s) located at this site. The membrane lining substance thus formed may play a role in stabilizing the squamous cell membranes, thereby maintaining the structural integrity of the epithelium against mechanical stress coming from the esophageal lumen.
Similar content being viewed by others
References
Barondes SH (1984) Soluble lectins: a new class of extracellular protein. Science 223:1259–1264
Cerra RF, Haywood-Reid PL, Barondes SH (1984) Endogenous mammalian lectin localized extracellularly in lung elastic fibers. J Cell Biol 98:1580–1589
Clerch LB, Whitney P, Hass M, Brew K, Miller T, Werner R, Massaro D (1988) Sequence of a full length cDNA for rat lung β-galactoside-binding protein: primary and secondary structure of the lectin. Biochemistry 27:692–699
Cooper DNW, Barondes SH (1990) Evidence for export of a muscle lectin from cytosol to extracellular matrix and for a novel secretory mechanism. J Cell Biol 110:1681–1691
Drickamer K (1988) Two distinct classes of carbohydrate-recognition domains in animal lectins. J Biol Chem 263:9557–9560
Gitt MA, Massa SM, Leffler H, Barondes SH (1992) Isolation and expression of a gene encoding L-14-II, a new human soluble lactose-binding lectin. J Biol Chem 267:10601–10606
Hashimoto K (1969) Cellular envelopes of keratinized cells of the human epidermis. Arch Klin Exp Dermatol 235:374–385
Hirabayashi J, Kasai KI (1988) Complete amino acid sequence of a β-galactoside-binding lectin from human placenta. J Biochem (Tokyo) 104:1–4
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Leffler H, Masiarz FR, Barondes SH (1989) Soluble lactosebinding vertebrate lectins: a growing famity. Biochemistry 28:9222–9229
Matoltsy AG, Parakkal PF (1965) Membrane coating granules of keratinizing epithelia. J Cell Biol 24:297–307
Oda Y, Leffler H, Sakakura Y, Kasai KI, Barondes SH (1991) Human breast carcinoma encoding a galactoside-binding lectin homologous mouse mac-2 antigen. Gene 99:279–283
Oda Y, Herrmann J, Gitt MA, Turck CW, Burlingame AL, Barondes SH, Leffler H (1993) Soluble lactose-binding lectin from rat intestine with two different carbohydrate-binding domains in the same peptide chain. J Biol Chem 268:5929–5939
Strauss JS, Matoltsy AG (1977) Skin. In: Weiss L, Greep RO (eds) Histology. McGraw-Hill, New York, pp 575–613
Tokuyasu KT (1973) A technique for ultracryotomy of cell suspensions and tissues. J Cell Biol 57:551–565
Towbin H, Stehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Wasano K, Hirakawa Y, Yamamoto T (1990) Immunohistochemical localization of 14 kDa β-galactoside-biding lectin in various organs of rat. Cell Tissue Res 259:43–49
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wasano, K., Hirakawa, Y. Rat intestinal galactoside-binding lectin L-36 functions as a structural protein in the superficial squamous cells of the esophageal epithelium. Cell Tissue Res 281, 77–83 (1995). https://doi.org/10.1007/BF00307960
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00307960