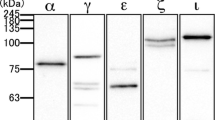
Abstract
We have localized at light and electron-microscopic level the growth-associated protein GAP-43 in adrenal gland using single and double labelling immunocytochemistry. Clusters of GAP-43-immunofluorescent chromaffin cells and many immunofluorescent fibres were observed in the medulla. GAP-43-immunoreactive fibres also formed a plexus under the capsule, crossed the cortex and ramified in the zona reticulata. Double labelled sections showed the coexpression of GAP-43 with a subpopulation of tyrosine hydroxylase-and of dopamine-β-hydroxylase-immunoreactive chromaffin cells. Dual colour immunofluorescence for GAP-43 and calcitonin gene-related peptide (CGRP) revealed that some of the GAP-43-immunoreactive fibres also express CGRP. Pre-embedding electron microscopy showed GAP-43 immunoreactivity associated with the plasma membranes and cytoplasm of noradrenaline-producing chromaffin cells, and with processes of nonmyelin-forming Schwann cells. Immunoreactive unmyelinated axons and terminals were also observed. The immunostained terminals made symmetrical synaptic contacts with chromaffin cells. Immunoreactive unmyelinated fibres and small terminals were present in the cortex. Our results show that GAP-43 is expressed in noradrenergic chromaffin cells and in various types of nerve fibres that innervate the adrenal. Likely origins for these fibres include preganglionic sympathetic fibres which innervate chromaffin cells, postganglionic sympathetic fibres in the cortex, and CGRP containing sensory fibres.
Similar content being viewed by others
References
Arvidsson U, Risling M, Cullheim S, Dagerlind Å, Lindå H, Shupliakov O, Ulfhake B, Hökfelt T (1992) On the distribution of GAP-43 and its relation to serotonin in adult monkey and cat spinal cord and lower brainstem. Eur J Neurosci 4:777–784
Averill S, Ching YP, Wilkin GP, López Costa JJ, Priestley JV (1993) The growth associated protein GAP-43 in adult rat dorsal root ganglion cells is confined to specific neuro-chemical populations. Proc 32 IUPS Congress, Glasgow, UK
Basi GS, Jacobson RD, Virag I, Schilling J, Skene JHP (1987) Primary structure and transcriptional regulation of GAP-43, a protein associated with nerve growth. Cell 49:785–91
Bendotti C, Servadio A, Samanin R (1991) Distribution of GAP-43 mRNA in the brain stem of adult rats as evidenced by in situ hybridization: localization within monoaminergic neurons. J Neurosci 11:600–607
Benowitz LI, Routtenberg A (1987) A membrane phosphoprotein associated with neural development, axonal regeneration, phospholipid metabolism, and synaptic plasticity. Trends Neurosci 10:527–532
Benowitz LI, Apostolides PJ, Perrone-Bizzozero NI, Finkelstein SP, Zwiers H (1988) Anatomical distribution of the growth-associated protein GAP-43/B-50 in the adult rat brain. J Neurosci 8:339–352
Benowitz LI, Rodriguez WR, Neve RL (1990) The pattern of GAP-43 immunostaining changes in the rat hippocampal formation during reactive synaptogenesis. Mol Brain Res 8: 17–23
Chong MS, Fitzgerald M, Winter J, Hu-Tsai M, Emson PC, Wiese U, Woolf CJ (1992) GAP-43 mRNA in rat spinal cord and dorsal root ganglia neurons: Developmental changes and re-expression following peripheral nerve injury. Eur J Neurosci 4: 883–895
Coggins PJ, Zwiers H (1991) B-50 (GAP-43): biochemistry and functional neurochemistry of a neuron-specific phosphoprotein. J Neurochem 56: 1095–1106
Costello B, Meymandi A, Freeman JA (1990) Factors influencing GAP-43 gene expression in PC12 pheochromocytoma cells. J Neurosci 10: 1398–1406
Coupland RE (1965a) Electron microscopic observations on the structure of the rat adrenal medulla. 1. The ultrastructure and organization of chromaffin cells in the normal adrenal medulla. J Anat 99: 231–254
Coupland RE (1965b) Electron microscopic observations on the structure of the rat adrenal medulla. 2. Normal innervation. J Anat 99: 255–272
Curtis R, Hardy R, Reynolds R, Spruce BA, Wilkin GP (1991) Down-regulation of GAP-43 during oligodendrocyte developmen and lack of expression by astrocytes in vivo: implications for macroglial differentiation. Eur J Neurosci 3: 876–886
Curtis R, Stewart HJS, Hall SM, Wilkin GP, Mirsky R (1992) GAP-43 is expressed by nonmyelin-forming Schwann cells of the peripheral nervous system. J Cell Biol 116: 1455–1464
Curtis R, Averill S, Priestley JV, Wilkin GP (1993) The distribution of GAP-43 in normal rat spinal cord. J Neurocytology 22: 39–50
Dekker LV, De Graan PNE, Oestreicher AB, Versteeg DHG, Gispen WH (1989) Inhibition of noradrenaline release by antibodies to B-50 (GAP-43). Nature 342: 74–76
Fuxe K, Goldstein M, Hökfelt T, Joh TH (1971) Cellular localization of dopamine-β-hydroxylase and phenylethanolamine N-methyltransferase as revealed by immunohistochemistry. In: Eranko O (ed) Histochemistry of nervous transmission. Prog Brain Res, vol. 34. Elsevier, Amsterdam, pp 127–138
Gorgels TGMF, Van Lookeren Campagne M, Oestreicher AB, Gribnau AAM, Gispen WH (1989) B-50/GAP-43 is localized at the cytoplasmic side of the plasma membrane in developing and adult rat pyramidal tract. J Neurosci 9: 3861–3869
Grant NJ, Konig F, Deloulme J.-C, Aunis D, Langley K (1992) Noradrenergic, but not adrenergic chromaffin cells in the adrenal gland express neuromodulin (GAP-43). Eur J Neurosci 4: 1257–1263
Hillarp NA, Hökfelt T (1954) Evidence of adrenaline and noradrenaline in separate adrenal medullary cells. Acta Physiol Scand 30: 55–68
Jap Tjoen San ER, Schmidt-Michels MH, Spruijt BM, Oestreicher AB, Schotman P, Gispen WH (1991) Quantitation of the growth-associated protein B-50/GAP-43 and neurite outgrowth in PC12 cells. J Neurosci Res 29: 149–154
Kesse WK, Parker TL, Coupland RE (1988) The innervation of the adrenal gland I. The source of pre-and postganglionic nerve fibres to the rat adrenal gland. J Anat 157: 33–41
Kleitman N, Holzwarth MA (1985) Catecholaminergic innervation of the rat adrenal cortex. Cell Tissue Res 241: 139–147
Kruger L, Bendotti C, Rivolta R, Samanin R (1993) The distribution of GAP-43 mRNA in the adult rat brain. J Comp Neurol (in press)
Kuramoto H, Kondo H, Fujita T (1987) Calcitonin gene-related peptide (CGRP)-like immunoreactivity in scattered chromaffin cells and nerve fibers in the adrenal gland of rats. Cell Tissue Res 247: 309–315
Lindā H, Piehl F, Dagerlind Å, Verge VMK, Arvidsson U, Cullheim S, Risling M, Ulfhake B, Hökfelt T (1992) Expression of GAP-43 mRNA in the adult mammalian spinal cord under normal conditions and after different types of lesions, with special reference to motoneurons. Exp Brain Res 91: 284–295
López Costa JJ, Averill S, Ching YP, Wilkin GP, Priestley JV (1993) Immunocytochemical localization of a growth associated protein (GAP-43) in adrenal gland. Proc 32 IUPS Congress, Glasgow, UK
Malvaldi G, Mencacci P, Viola-Magni MP (1968) Mitoses in the adrenal medullary cells. Experientia 24: 475–476
Mazzoni IE, Jaffe E, Cuello AC (1991) Production and immunocytochemical application of a highly sensitive and specific monoclonal antibody against rat dopamine-β-hydroxylase. Histochemistry 96: 45–50
Meiri KF, Pfenninger KH, Willard MB (1986) Growth-associated protein, GAP-43, a polypeptide that is induced when neurons extend axons, is a component of growth cones and corresponds to pp46, a major polypeptide of a subcellular fraction enriched in growth cones. Proc Natl Acad Sci USA 83: 3537–3541
Merighi A, Polak JM, Gibson SJ, Gulbenkian S, Valentino KL, Peirone SM (1988) Ultrastructural studies on calcitonin gene-related peptide-, tachykinins-and somatostatin-immunoreactive neurons in rat dorsal root ganglia: evidence for the colocalization of different peptides in single secretory granules. Cell Tissue Res 254: 101–109
Nelson RB, Friedman DP, O'Neill JB, Mishkin M, Routtenberg A (1987) Gradients of protein kinase C substrate phosphorylation in primate visual system peak in visual memory storage areas. Brain Res 416: 387–392
Neve RL, Finch EA, Bird ED, Benowitz LI (1988) The growth-associated protein GAP-43 (B-50, F1) is expressed selectively in associative regions of the adult human brain. Proc Natl Acad Sci USA 85: 3638–3642
Priestley JV, ALvarez FJ, Averill S (1992) Pre-embedding electron microscopic immunocytochemistry. In: Polak JM, Priestley JV (eds) Electron microscopic immunocytochemistry. Oxford University Press, Oxford, pp 89–121
Purves D, Voyvodic JT (1987) Imaging mammalian nerve cells and their connections over time in living animals. TINS 10: 398–404
Purves D, Hadley RD, Voyvodic JT (1986) Dynamic changes in the dendritic geometry of individual neurons visualized over periods of up to three months in the superior cervical ganglion of living mice. J Neurosci 6: 1051–1060
Rosenthal A, Chan SY, Henzel W, Haskell C, Kuang W-J, Chen JN, Wilcox A, Ullrich DV, Goeddel E, Routtenberg A (1987) Primary structure and mRNA localization of protein F1, a growth-related protein kinase C substrate associated with synaptic plasticity. EMBO J 6: 3641–3646
Schmidt RE, Spencer SA, Coleman BD, Roth KA (1991) Immunohistochemical localization of GAP-43 in rat and human sympathetic nervous system-effects of aging and diabetes. Brain Res 552: 190–197
Schreyer DJ, Skene JHP (1991) Fate of GAP-43 in ascending spinal axons of DRG neurons after peripheral nerve injury: delayed accumulation and correlation with regenerative potential. J Neurosci 11: 3738–3751
Semenenko FM, Cuello AC, Goldstein M, Lee KY, Sidebottom E (1986) A monoclonal antibody against tyrosine hydroxylase: application in light and electron microscopy. J Histochem Cytochem 34: 817–821
Skene JHP (1989) Axonal growth-associated proteins. Ann Rev Neurosci 12: 127–156
Skene JHP, Jacobson RD, Snipes GJ, Mc Guire CB, Norden JJ, Freeman JA (1986) A protein induced during nerve growth (GAP-43) is a major component of growth-cone membranes. Science 233: 783–786
Stewart HJS, Cowen T, Curtis R, Wilkin GP, Mirsky R, Jessen KR (1992) GAP-43 immunoreactivity is widespread in the autonomic neurons and sensory neurons of the rat. Neuroscience 47: 673–684
Van Lookeren Campagne M, Oestreicher AB, Van Bergen En Henegouwen PMP, Gispen WH (1989) Ultrastructural immunocytochemical localisation of B-50/GAP-43, a protein-kinase C substrate, in isolated presynaptic nerve terminals and neuronal growth cones. J Neurocytology 18: 479–489
Verge VMK, Tetzlaff W, Richardson PM, Bisby MA (1990) Correlation between GAP-43 and nerve growth factor receptors in rat sensory neurons. J Neurosci 10: 926–934
Verhaagen J, Van Hooff COM, Edwards PM, De Graan PNE, Oestreicher AB, Schotman P, Jennekens FGI, Gispen WH (1986) The kinase C substrate protein B-50 and axonal regeneration. Brain Res Bull 17: 737–741
Voyvodic JT (1989) Peripheral target regulation of dendritic geometry in the rat superior cervical ganglion. J Neurosci 9: 1997–2010
Wessel TC, Joh TH (1992) Parallel upregulation of catecholaminesynthesizing enzymes in rat brain and adrenal gland: effects of reserpine and correlation with immediate early gene expression. Mol Brain Res 15: 349–360
Woolf CJ, Reynolds ML, Chong MS, Emson P, Irwin N, Benowitz LI (1992) Denervation of the motor endplate results in the rapid expression by terminal Schwann cells of the growth-associated protein GAP-43. J Neurosci 12: 3999–4010
Yao GL, Kiyama H, Tohyama M (1993) Distribution of GAP-43 (B50/F1) mRNA in the adult rat brain by in situ hybridization using an alkaline phosphatase labelled probe. Mol Brain Res 18: 1–16
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Costa, J.J.L., Averill, S., Ching, Y.P. et al. Immunocytochemical localization of a growth-associated protein (GAP-43) in rat adrenal gland. Cell Tissue Res 275, 555–566 (1994). https://doi.org/10.1007/BF00318824
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318824