Summary
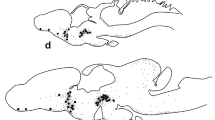
The peptidergic system in the optic ganglia of Astacus leptodactylus is characterized by the immunocytochemical application of 15 antisera raised against biologically active peptides of vertebrates and invertebrates. Positive reactions were found with anti-FMRFamide, antiαMSH, anti-vasotocin, anti-gastrin, anti-CCK, anti-oxytocin, anti-secretin, anti-glucagon and anti-GIP. Based on immunochemical reaction and localization it is possible to distinguish 30 cell groups. Only part of these cell groups is found in the known classical neurosecretory cell regions. This observation demonstrates a more extensive peptidergic system than formerly recognized. The morphology of this peptidergic system suggests that one part is neurohormonal and the other part neurotransmitter-like or neuromodulatory.
Similar content being viewed by others
References
Bliss DE, Durand JB, Welsh JH (1954) Neurosecretory systems in Decapod Crustacea. Z Zellforsch 39:530–536
Bodenmüller H, Schaller HC (1981) Conserved aminoacid sequence of a neuropeptide, the head activator, from coelenterates to humans. Nature 293:579–580
Boer HH, Schot LPC (1983) Phylogenetic aspects of peptidergic systems. In: Lever J, Boer HH (eds) Molluscan neuro-endocrinology. Mon Kon Akad Wet, North-Holland Publ Co, Amsterdam Oxford New York
Boer HH, Schot LPC, Roubos EW, Maat A ter, Lodder JC, Rei-chelt D, Swaab DF (1979) ACTH-like immunoreactivity in two electrotonically coupled giant neurons in the pond snail Lymnaea stagnalis. Cell Tissue Res 202:231–240
Boer HH, Schot LPC, Veenstra JA, Reichelt D (1980) Immunocytochemical identification of neural elements in the central nervous system of a snail, some insects, a fish and a mammal with an antiserum to the molluscan cardio-excitatory tetrapeptide FMRF-amide. Cell Tissue Res 213:21–27
Bullock TH, Horridge GA (eds) (1965) Structure and function in the nervous systems of Invertebrates. WH Freeman and Co, San Francisco London, Vol I, vol II
Durand JB (1956) Neuroscretory cell types and their secretory activity in the crayfish. Biol Bull 111:62–76
Duve H, Thorpe A (1979) Immunofluorescent localization of insulin-like material in the median neuroscretory cells of the blowfly Calliphora vomitoria (Diptera). Cell Tissue Res 200:187–191
Duve H, Thorpe A (1980a) Isolation and localization of an insect insulin-like material: immunological, biological and physical characteristics. Gen Comp Endocrinol 40:363–364
Duve H, Thorpe A (1980b) Localization of pancreatic polypeptide (PP)-like immunoreactive material in the neurons of the brain of the blowfly Calliphora erythrocephala (Diptera). Cell Tissue Res 210:101–109
Duve H, Thorpe A (1981) Gastrin/CCK-like immunoreactive neurons in the brain of the blowfly Caliphora erythrocephala (Diptera). Gen Comp Endocrinol 43:387–391
Duve H, Thorpe A, Lazarus NR (1979) Isolation of material displaying insulin-like immunological and biological activity from the brain of the blowfly Calliphora vomitoria. Biochem J 184:221–227
Elofsson R, Klemm N (1972) Monoamine-containing neurons in the optic ganglia of crustaceans and insects. Z Zellforsch 133:475–499
Elofsson R, Nässel D, Myhrberg H (1977) A catecholaminergic neuron connecting the first two optic neuropiles (lamina ganlionaris and medulla externa) of the crayfish Pacifastacus leniusculus. Cell Tissue Res 182:287–297
Enami M (1951) The sources and activities of two chromatophorotropic hormones in crabs of the genus Sesarma. II. Histology of incretory elements. Biol Bull Woods Hole 101:241–258
Gorgels-Kallen JL, Van Herp F (1981) Localization of crustacean hyperglycemic hormone (CHH) in the X-organ sinus gland complex in the eyestalk of the crayfish Astacus leptodactylus (Nordmann 1842). J Morphol 170:347–355
Gorgels-Kallen JL, Voorter CEM (1984) Secretory stages of individual CHH producing cells in the eyestalk of the crayfish Astacus leptodactylus, determined by means of immunocytochemistry. Cell Tissue Res 237:291–298
Grimmelikhuyzen CJP, Balfe A, Emson PC, Powell D, Sundler F (1981) Substance-P-like immunoreactivity in the nervous system of hydra (Hydra attenuata). Histochemistry 71:325–334
Hamori J, Horridge GA (1966a) The lobster optic lamina. I. General organization. J Cell Sci 1: 249–256
Hamori J, Horridge GA (1966b) The lobster optic lamina. II. Types of synapse. J Cell Sci 1:257–270
Hanström B (1924) Untersuchungen über das Gehirn, insbesondere die Sehganglien der Crustaceen. Ark Zool 16:1–119
Hisano S (1974) The eyestalk neurosecretory cell types in the freshwater prawn Palaemon paucidens. I. A light microscopical study. J Fac Sci Hokkaido Univ Ser VI Zool 19:503–516
Jacobs AAC, Van Herp F (1984) Immunocytochemical localization of a substance in the eyestalk of the prawn Palaemon serratus, reactive with an anti-FMRF-amide rabbit-serum. Cell Tissue Res 235:601–605
Kleinholz LH, Keller R (1979) Endocrine regulation in Crustacea. In: Barrington EJW (ed) Hormones and evolution. Academic Press, New York San-Francisco London, vol I, ch IV
Loumaye E, Thorner J, Catt KJ (1982) Yeast mating pheromone activates mammalian gonadotrophs: evolutionary conservation of a reproductive hormone. Science 218:1323–1325
Mantillas JR, McGinty JF, Selverston AJ, Karten H, Bloom FE (1981) Immunocytochemical localization of enkephalin and substance P in retina and eyestalk neurones of lobster. Nature 293:576–578
Pearse AGE, Polak JM (1975) Bifunctional reagents as vaporphase and liquid-phase fixatives for immunochemistry. Histochem J 7:179–186
Scharrer B (1978) Peptidergic neurons: facts and trends. Gen Comp Endocrinol 34:50–62
Schot LPC, Boer HH, Swaab DF, Van Noorden S (1981) Immunocytochemical demonstration of peptidergic neurons in the central nervous system of the pond snail Lymnaea stagnalis with anti-sera raised to biologically active peptides of vertebrates. Cell Tissue Res 216:273–291
Schot LPC, Boer HH, Montagne-Wajer C (1984) Characterization of multiple immunoreactive neurons in the central nervous system of the pond snail Lymnaea stagnalis with different fixatives and antisera adsorbed with the homologous and the heterologous antigens. In: Schot LPC (ed) Immunocytochemical studies on peptidergic neurons in the pond snail Lymnaea stagnalis. Thesis, Free University Amsterdam
Sternberger LA (1979) Immunocytochemistry, 2nd, ed, John Wiley & Sons, New York Chicester Brisbane Toronto
Strolenberg GECM (1979) Functional aspects of the sinus gland in the neurosecretory system of the crayfish Astacus leptodactylus. An ultrastructural approach. Thesis Fac Science, Catholic University, Nijmegen, The Netherlands
Swaab DF, Pool LW, Van Leeuwen F (1977) Can specificity ever be proven in immunocytochemical staining? J Histochem Cytochem 25:388–391
Van Herp F, Van Buggenum HJM (1979) Immunocytochemical localization of hyperglycemic hormone (HGH) in the neurosecretory system of the eyestalk of the crayfish Astacus leptodactylus. Experientia 35:1527–1528
Van Herp F, Bellon-Humbert C (1982) Localisation immunocytochimique de substances apparentées à la neurophysine et à la vasopressine dans le pédoncule oculaire de Palaemon serratus Pennant (Crustacé Décapode Natantia). CR Acad Sci Paris 295:97–102
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Van Deijnen, J.E., Vek, F. & Van Herp, F. An immunocytochemical study of the optic ganglia of the crayfish Astacus leptodactylus (Nordmann 1842) with antisera against biologically active peptides of vertebrates and invertebrates. Cell Tissue Res. 240, 175–183 (1985). https://doi.org/10.1007/BF00217572
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00217572