Summary
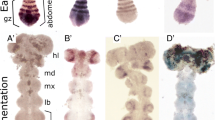
Drosophila embryos, exposed to ether between 1 and 4 h after oviposition, develop defects ranging from the complete lack of segmentation to isolated gaps in single segments. Between these extremes are varying extents of incomplete and abnormal segmentation. On the basis of both their temporal and spatial characteristics, five major phenotype classes may be distinguished: headless — unsegmented or incompletely segmented anteriorly; gap — interruptions of segmentation not obviously periodic; alternating segment gaps — interruptions with double segment periodicities; fused segments; and short segments — truncations with single segment periodicities. Many defects resemble known mutant phenotypes. The disturbances in segmentation are predominantly global and frequently accompanied by alterations in segment specification, such that the segments obtained show no resemblance to the normal homologues. These features, together with the distinctive spatiotemporal characteristics of the defects, all point to segmentation as a dynamic process. The regular spacing of the segments and the fact that the entire range of defects is inducible by ether are further consistent with the hypothesis that at least part of the segmentation process may consist of physicochemical reactions coordinated over the whole body. The relationship between our data and data from genetic and other analyses are briefly discussed.
Similar content being viewed by others
References
Cooke J, Zeeman C (1976) A clock and wavefront model for the control of the number of repeated structures during animal morphogenesis. J Theor Biol 58:455–476
Coulter D, Wieschaus E (1986) Segmentation genes and the distributions of transcripts. Nature 321:472–474
Elsdale T, Pearson M, Whitehead M (1976) Abnormalities in somite segmentation following heat-shock to Xenopus embryos. J Embryol Exp Morphol 35:625–635
Frohnhöfer HG, Lehmann R, Nüsslein-Volhard C (1986) Manipulating the anteroposterior pattern of the Drosophila embryo. J Embryol Exp Morphol [suppl] 97:169–179
Gloor H (1947) Phanokopie-Versuche mit Äther an Drosophila. Rev Suisse Zool 54:637–712
Goodwin BC, Skelton JL, Kirk-Bell SM (1983) Control of regeneration and morphogenesis by divalent cations in Acetabularia mediterranea. Planta 157:1–7
Harding K, Rushlow C, Doyle HJ, Hoey T, Levine M (1986) Cross-regulatory interactions among pair-rule genes in Drosophila. Science 233:953–959
Ho MW (1984) Environment and heredity in development and evolution. In: Ho MW, Saunders PT (eds) Beyond neo-darwinism: an introduction to the new evolutionary paradigm. Academic Press, London, pp 267–289
Ho MW, Tucker C, Keeley D, Saunders PT (1983a) Effects of successive generations of ether treatment on penetrance and expression of the bithorax phenocopy in Drosophila melanogaster. J Exp Zool 225:357–368
Ho MW, Bolton E, Saunders PT (1983b) The bithorax phenocopy and pattern formation. I. Spatiotemporal characteristics of the phenocopy response. Expl Cell Biol 51:282–290
Ho MW, Saunders PT, Bolton E (1983c) The bithorax phenocopy and pattern formation. II. A model of prepattern formation. Expl Cell Biol 51:291–299
Jaffe LF (1979) On the centripetal course of development, the Fucus egg and self-electrophoresis. In: Language, communications and development. Dev Biol [Suppl 3] pp 83–111
Johnson SM, Bangham AD (1969) The action of anaesthetics on phospholipid membranes. Biochim Biophys Acta 193:92–104
Jürgens G, Wieschaus E, Nüsslein-Volhard C, Kluding H (1984) Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. II. Zygotic loci on the third chromosome. Wilhelm Roux's Arch 193:283–295
Kauffman SA, Shymko RM, Trabert K (1978) Control of sequential compartment formation in Drosophila. Science 199:259–269
Kilchherr F, Baumgartner S, Bopp D, Frei E, Noll M (1986) Isolation of the paired gene of Drosophila and its spatial expression during early embryogenesis. Nature 321:493–499
Lohs-Schardin M, Cremer C, Nüsslein-Volhard C (1979) A fate map of the larval epidermis of Drosophila melanogaster. Localized defects following irradiation of the blastoderm with an ultraviolet laser microbeam. Dev Biol 73:239–255
Martinez-Arias A (1986) A molecular season for descriptive embryology. Trends Genet 2:146
Mee J, French V (1986) Disruption of segmentation in a short germ insect embryo. II. The structure of segmental abnormalities induced by heat shock. J Embryol Exp Morphol 96:267–294
Mohler J, Wieschaus EF (1986) Dominant maternal-effect mutations of Drosophila melanogaster causing the production of double abdomen embryos. Genetics 112:803–822
North G (1986) Descartes and the fruitfly. Nature 322:404–405
Nüsslein-Volhard C (1977) Genetic analysis of pattern-formation in the embryo of Drosophila melanogaster. Characterization of the maternal-effect mutant bicaudal. Wilhelm Roux's Arch 183:249–268
Nüsslein-Volhard C, Wieschaus E (1980) Mutations affecting segment number and polarity in Drosophila. Nature 287:795–801
Nüsslein-Volhard C, Wieschaus E, Kluding H (1984) Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. I. Zygotic loci on the second chromosome. Wilhelm Roux's Arch 193:267–282
Sander K (1976) Specification of the basic body pattern in insect embryogenesis. Adv Insect Physiol 12:125–238
Sander K (1984) Embryonic pattern formation in insects: Basic concepts and their experimental foundations. In: Malacinski GM, Bryant SV (eds) Pattern formation. Macmillan, New York, pp 245–268
Saunders PT, Ho MW (1985) Primary and secondary waves in prepattern formation. J Theor Biol 114:491–504
Schubiger G (1976) Adult differentiation from partial Drosophila embryos after egg ligation during stages of nuclear multiplication and cellular blastoderm. Dev Biol 50:476–488
Schüpbach T, Wieschaus E (1986) Maternal effect mutations altering the anterior-posterior pattern of the Drosophila embryo. Roux's Arch Dev Biol 195:302–317
Steiner E (1976) Establishment of compartments in the developing leg imaginal discs of Drosophila melanogaster. Wilhelm Roux's Arch 180:9–30
Struhl G (1983) Role of the esc gene product in ensuring the selective expression of segment-specific homeotic genes in Drosophila. J Embryol Exp Morphol 76:297–331
Turner FRS, Mahowald AP (1979) Scanning electron microscopy of Drosophila melanogaster embryogenesis: III. Formation of the head and caudal segments. Dev Biol 68:96–109
Van der Meer JM (1977) Optical clear and permanent whole mount preparations for phase-contrast microscopy of cuticular structures of insect larvae. Drosophila Inf Serv 52:160
Weir MP, Kornberg T (1985) Pattern of engrailed and fushi tarazu transcripts reveal novel intermediate stages in Drosophila segmentation. Nature 318:433–445
Wieschaus E, Nüsslein-Volhard C, Jürgens G (1984) Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. III. Zygotic loci on the X-chromosome and fourth chromosome. Wilhelm Roux's Arch 193:296–307
Wieschaus E, Gehring W (1976) Clonal analysis of primordial disc cells in the early embryo of Drosophila melanogaster. Dev Biol 50:249–263
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ho, M.W., Matheson, A., Saunders, P.T. et al. Ether-induced segmentation disturbances in Drosophila melanogaster . Roux's Arch Dev Biol 196, 511–521 (1987). https://doi.org/10.1007/BF00399875
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00399875