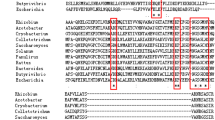
Abstract
The synthesis of 5-aminolevulinic acid commences with the ligation of glutamate to a specific tRNAGlu by a glutamyl-tRNA synthetase (E.C. 6.1.1.17) (Huang et al., 1984, Science 225, 1482–1484). The synthetase from the yellow pigment mutant C-2A′ of the unicellular green alga Scenedesmus obliquus was purified by sequential column chromatography on Sephacryl S-300, Blue Sepharose, phosphocellulose P11 and by fast protein liquid chromatography (FPLC) on Mono Q. After denaturing sodium dodecylsulfate (SDS)-gel electrophoresis the purified enzyme preparation revealed a single protein band with a molecular mass of 55 kDa, proving the apparent homogeneity of the glutamyl-tRNA synthetase. A molecular mass of 105 ± 10 kDa was determined for the native protein by chromatography on Sephadex G-150. From these data it can be concluded that the glutamyl-tRNA synthetase from S. obliquus is a homodimer. The purified protein is active within a pH range from 7.0 to 9.0 with a maximum activity at pH 8.0. Kinetics for the binding of glutamate to the tRNA, performed with highly purified enzyme preparations, showed a K m value of 2.3 μM ± 0.3 for glutamate.
Similar content being viewed by others
Abbreviations
- ALA:
-
5-aminolevulinic acid
- FPLC:
-
fast protein liquid chromatography
- Glu:
-
glutamate
- Hepes:
-
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- SDS:
-
sodium dodecylsulfate
- Tricine:
-
N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]-glycine
References
Avissar Y.S., Beale, S.I. (1988) Biosynthesis of tetrapyrrole pigment precursors: Formation and utilization of glutamyl-tRNA for δ-aminolevulinic acid synthesis by isolated enzyme fractions from Chlorella vulgaris. Plant Physiol. 88, 879–886
Blum, H., Beier, H., Gross, H.J. (1987) Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8, 93–99
Beale, S.I. (1990) Biosynthesis of the tetrapyrrole pigment precursor, δ-aminolevulinic acid, from glutamate. Plant Physiol. 93, 1273–1279
Bishop, N.I. (1971) Preparation and properties of mutants: Scenedesmus. Methods Enzymol. 23, 130–143
Bradford, M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principles of protein-dye binding. Anal. Biochem. 72, 248–254
Breu, V., Dörnemann D. (1988) Formation of 5-aminolevulinate via glutamate-1-semialdehyde and 4,5-dioxovalerate with participation of an RNA component in Scenedesmus obliquus mutant C-2A′. Biochim. Biophys. Acta 967, 135–140
Bryant, P., Kannangara, C.G. (1987) Biosynthesis of δ-aminolevulinate in greening barley leaves.VIII. Purification and characterization of the glutamyl-tRNA-ligase. Carlsberg Res. Commun. 52, 99–109
Chang, T.-E., Wegmann, B., Wang, W.-Y. (1990) Purification and characterization of glutamyl-tRNA synthetase. Plant Physiol. 93, 1641–1649
Chen, M.-W., Jahn, D., Schön, A., O'Neill, G.P., Söll, D. (1990) Purification and characterization of Chlamydomonas reinhardtii chloroplast glutamyl-tRNA synthetase, a natural misacylating enzyme. J. Biol. Chem. 265, 4054–4057
Dörnemann, D. (1992) New aspects of the intermediates, catalytic components and the regulation of the C5-pathway to chlorophyll. In: Regulation of chloroplast biogenesis, pp 175–181, Argyroudi-Akoyunoglou, J.H., ed. Plenum Press, New York
Gaffron, H. (1939) Reduction of carbon monoxide with molecular hydrogen in green algae. Nature 143, 204–205
Huang, D.-D., Wang, W.-Y., Gough, S.P., Kannangara, C.G. (1984) δ-aminolevulinic acid-synthesizing enzymes need RNA moiety for activity. Science 225, 1482–1484
Jahn, D., O'Neill, G.P., Verkamp, E., Söll, D. (1992) Glutamate tRNA: Involvement in protein synthesis and aminolevulinate formation in Chlamydomonas reinhardtii. Plant Physiol. Biochem. 30, 245–253
Kannangara, C.G., Gough, S.P., Oliver, R.P., Rasmussen, S.K. (1984) Biosynthesis of δ-aminolevulinate in greening barley leaves. VI. Activation of glutamate by ligation to RNA. Carlsberg Res. Commun. 49, 417–437
Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685
Moras, D. (1990) Structural and functional relationships between aminoacyl tRNA synthetases. Trends Biochem. Sci. 17, 159–164
Ratinaud, M.J., Thomes, J.C., Julien, R. (1983) Glutamyl-tRNA synthetases from wheat. Isolation and characterization of three dimeric enzymes. Eur. J. Biochem. 135, 471–477
Rieble, S., Beale, S.I. (1989) Separation of the enzymes required for transformation of glutamate to δ-aminolevulinic acid in extracts of Synechocystis sp. PCC 6803. Plant Physiol. 89, S-51
Rüdiger, W., Schoch, S. (1988) Chlorophylls. In: Plant pigments, pp. 1–59, Goodwin, T.W., ed. Academic Press, London, San Diego
Senger, H., Bishop, N.I. (1972) The development of structure and function in chloroplasts of greening mutants of Scenedesmus I. Formation of chlorophyll. Plant Physiol. 13, 633–649
Senger, H., Mell, V. (1977) Preparation of photosynthetically active particles from synchronized cultures of unicellular green algae. Methods Cell Biol. 15, 201–214
Thomes, J.C., Ratinaud, M.H., Julien, R. (1983) Dimeric glutamyl-tRNA synthetases from wheat. Kinetic properties and functional structures. Eur. J. Biochem. 135, 479–484
Wang, W.-Y., Gough, S.P., Kannangara, C.G. (1981) Biosynthesis of δ-aminolevulinate in greening barley leaves. IV. Isolation of three soluble enzymes required for the conversion of glutamate to δ-aminolevulinate. Carlsberg Res. Commun. 46, 243–257
Author information
Authors and Affiliations
Additional information
This work was supported by a grant of the Deutsche Forschungsgemeinschaft. U.C. Vothknecht is grateful for a Nachwuchs-förderungsstipendium des Landes Hessen. The authors want to thank Ms. B. Böhm, J. Gade and K. Eckhardt for skillful technical assistance. The authors also want to thank Dr. C.G. Kannangara (Carlsberg Institute, Kopenhagen, Denmark) for the donation of tRNA from barley and Dr. D. Jahn (FB Biology/Microbiology, Philipps-University, Marburg, FRG) for the tRNAGlufrom E. coli.
Rights and permissions
About this article
Cite this article
Vothknecht, U.C., Senger, H. & Dörnemann, D. Purification and partial characterization of a glutamyl-tRNA synthetase from the unicellular green alga Scenedesmus obliquus, mutant C-2A′. Planta 192, 256–260 (1994). https://doi.org/10.1007/BF00194460
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00194460