Abstract
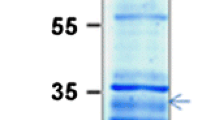
Thioredoxin (Td) f from pea (Pisum sativum L.) leaves was purified by a simple method, which provided a high yield of homogeneous Td f. Purified Td f had an isoelectric point of 5.4 and a relative molecular mass (Mr) of 12 kilodaltons (kDa) when determined by filtration through Superose 12, but an Mr of 15.8 kDa when determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The purified protein remained fully active for several months when conserved frozen at — 20° C. The pea protein was able to activate fructose1,6-bisphosphatase (FBPase; EC 3.1.3.11), but in contrast to other higher-plant Td f proteins, was not functional in the modulation of NADP+-malate dehydrogenase activity. In spite of the absence of immunological cross-reactions of pea and spinach Td f proteins with the corresponding antibodies, pea Td f activated not only the homologous FBPase, but also the spinach enzyme. The saturation curves for pea FBPase, either with fructose-1,6-bisphosphate in the presence of different concentrations of homologous Td f, or with pea Td f in the presence of excess substrate, showed sigmoid kinetics; this can be explained on the basis of a random distribution of fructose-1,6-bisphosphate, and of the oxidized and reduced forms of the activator, among the four Td f- and substrate-binding sites of this tetrameric enzyme. From the saturation curves of pea and spinach Td f proteins against pea FBPase, a 4:1 stoichiometry was determined for the Td f-enzyme binding. This is in contrast to the 2:1 stoichiometry found for the spinach FBPase. The UV spectrum of pea Td f had a maximum at 277 nm, which shifted to 281 nm after reduction with dithiothreitol (s at 280 nm for 15.8-kDa Mr = 6324 M−1 · cm−1). The fluorescence emission spectrum after 280-nm excitation had a maximum at 334 nm, related to tyrosine residues; after denaturation with guanidine isothiocyanate an additional maximum appeared at 350 nm, which is concerned with tryptophan groups. Neither the native nor the denatured form showed a significant increase in fluorescence after reduction by dithiothreitol, which means that the tyrosine and tryptophan groups in the reduced Td f are similarly exposed. Pea Td f appears to have one cysteine residue more than the three cysteines earlier described for spinach and Scenedesmus Td f proteins.
Similar content being viewed by others
Abbreviations
- DDT:
-
dithiothreitol
- ELISA:
-
enzyme-linked immunosorbent assay
- FBPase:
-
fructose- 1,6-bisphosphatase
- kDa:
-
kilodalton
- Mr :
-
relative molecular mass
- SDS-PAGE:
-
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- Td:
-
thioredoxin
Referencess
Ashton, A.R., Brennan, T., Anderson, L.E. (1980) Thioredoxin-like activity of thylakoid membranes. Plant Physiol. 60, 605–608
Buc, J., Riviere, M., Gontero, B., Sauve, P., Meunier, J.C., Ricard, J. (1984) Affinity chromatography on fructose-bisphosphatase-Sepharose, of two chloroplastic thioredoxins f. Purification and comparative molecular properties. Eur. J. Biochem. 140, 199–202
Buchanan, B.B. (1980) Role of light in the regulation of chloroplast enzymes. Annu. Rev. Plant Physiol. 31, 341–374
Buchanan, B.B., Wolosiuk, R.A., Schürmann, P. (1979) Thioredoxin and enzyme regulation. Trends Biochem. Sci. 4, 93–96
Carrasco, J.L., Chueca, A., Sahrawy, M., Hermoso, R., Lázaro, J.J., López Gorgé, J. (1992) Role of light in the in vivo and in vitro synthesis of spinach thioredoxin f. Physiol. Plant. 84, 236–242
Chardot, T., Meunier, J.C. (1990) Fructose-1,6-bisphosphatase and calcium activate oxidized spinach (Spinacia oleracea) chloroplast fructose-1,6-bisphosphatase. Plant Sci. 70, 1–9
Chueca, A., Sahrawy, M., Carrasco, J.L., Ramos, J.L., Lázaro, J.J., Hermoso, R., López Gorgé, J. (1990) In vivo synthesis and immunological relationship of thioredoxin f from pea and spinach. In: Current research in photosynthesis, vol. IV, pp. 163–166, Baltscheffsky, M., ed., Kluwer Academic Publishers, Dordrecht, The Netherlands
Clancey, C.J., Gilbert, H.F. (1987) Thiol/disulfide exchange in the thioredoxin-catalyzed reductive activation of spinach chloroplast fructose-1,6-bisphosphatase. J. Biol. Chem. 262, 13545–13549
Crawford, N.A., Droux, M., Kosower, N.S., Buchanan, B.B. (1989) Evidence for function of the ferredoxin/thioredoxin system in the reductive activation of target enzymes of isolated intact chloroplasts. Arch. Biochem. Biophys. 271, 223–239
Crawford, N.A., Yee, B.C., Hutcheson, S.W., Wolosiuk, R.A., Buchanan, B.B. (1986) Enzyme regulation in C4 photosnythesis: Purification, properties, and activities of thioredoxins from C4 and C3 plants. Arch. Biochem. Biophys. 244, 1–15
Cséke, C., Buchanan, B.B. (1986) Regulation of the formation and utilization of photosynthate in leaves. Biochim. Biophys. Acta 853, 43–64
Droux, M., Jacquot, J.P., Miginiac-Maslow, M., Gadal, P., Huet, J.C., Crawford, N.A., Yee, B.C., Buchanan, B.B. (1987) Ferredoxin thioredoxin reductase, an iron-sulfur enzyme linking light to enzyme regulation in oxygenic photosnythesis. Purification and properties of the enzyme from C-3, C-4 and cyanobacterial species. Arch. Biochem. Biophys. 252, 426–439
Eklund, H., Gleason, F.K., Holmgren, A. (1991) Structural and functional relations among thioredoxins of different species. Proteins Struct. Funct. Genet. 11, 13–28
Galmiche, J.M., Girault, G., Berger, G., Jacquot, J.P., Miginiac-Maslow, M., Wollman, E. (1990) Induction by different thioredoxins of ATPase activity in coupling factor 1 from spinach chloroplasts. Biochimie 72, 25–32
Häberlein, L., Schimpff-Weiland, G., Follmann, H. (1985) Unexpected specificity in the thioredoxin activation of fructose-bisphosphatases from different plants. Biochem. Biophys. Res. Commun. 127, 401–406
Hartman, H., Syvanen, M., Buchanan, B.B. (1990) Contrasting evolutionary histories of chloroplast thioredoxins f and m. Mol. Biol. Evol. 7, 247–254
Hermoso, R., De Felipe, M.R., Vivó, A., Chueca, A., Lázaro, J.J., López Gorgé, J. (1989) Immunogold localization of photosynthetic fructose-1,6-bisphosphatase in pea leaf tissue. Plant Physiol. 89, 381–385
Hermoso, R., Fonollá, J., De Felipe, M.R., Vivó, A., Chueca, A., Lázaro, J.J., López Gorgé, J. (1992) Double immunogold localization of thioredoxin f and photosynthetic fructose-1,6-bisphos-phatase in spinach leaves. Plant Physiol. Biochem. 30, 39–46
Hertig, C., Wolosiuk, R.A. (1980) A dual effect of Ca2+ on chloroplast fructose-1,6-bisphosphatase. Biochem. Biophys. Res. Commun. 97, 325–333
Hirs, C.H.W. (1967) Determination of cystine as cysteic acid. Methods Enzymol. 11, 59–62
Holmgren, A. (1985) Thioredoxin. Annu. Rev. Biochem. 54, 237–271
Hopper, S., Iurlano, D. (1983) Properties of a thioredoxin purified from rabbit bone marrow which fails to serve as a hydrogen donor for the homologous ribonucleotide reductase. J. Biol. Chem. 258, 13453–13457
Huppe, H.C., de Lamotte-Guéry, F., Jacquot, J.P., Buchanan, B.B. (1990) The ferredoxin-thioredoxin system of a green alga, Chlamydomonas reinhardtii. Identification and characterization of thioredoxins and ferredoxin-thioredoxin reductase components. Planta 180, 341–351
Hutcheson, S.W., Buchanan, B.B. (1983) Enzyme regulation in crassulacean acid metabolism photosynthesis. Studies on the ferredoxin/thioredoxin system of Kalanchoë daigremontiana. Plant Physiol. 72, 870–876
Jacquot, J.P. (1984) Post-translational modifications of proteins in higher plant chloroplasts: enzyme regulation by thiol-disulfide interchange. Physiol. Vég. 22, 487–507
Jacquot, J.P., Vidai, J., Gadal, P., Schürmann, P. (1978) Evidence for the existence of several enzyme-specific thioredoxins in plants. FEBS Lett. 96, 243–246
Jacquot, J.P., Buchanan, B.B., Martin, F., Vidal, J. (1981) Enzyme regulation in C4 photosynthesis. Purification and properties of thioredoxin-linked NADP-malate dehydrogenase from corn leaves. Plant Physiol. 68, 300–304
Johnson, T.C., Crawford, N.A., Buchanan, B.B. (1984) Thioredoxin system of the photosynthetic anaerobe Chromatium vinosum. J. Bacteriol. 158, 1061–1069
Johnson, T.C., Qiang Cao, R., Kung, J.E., Buchanan, B.B. (1987) Thioredoxin and NADP-thioredoxin reductase from cultured carrot cells. Planta 171, 321–331
Kamo, M., Tsugita, A., Wiessner, Ch., Wedel, N., Bartling, D., Herrmann, R.G., Aguilar, F., Gardet-Salvi, L., Schürmann, P. (1989) Primary structure of spinach-chloroplast thioredoxin f. Protein sequencing and analysis of complete cDNA clones for spinach-chloroplast thioredoxin f. Eur. J. Biochem. 182, 315–322
Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685
Langlotz, P., Wagner, W., Follmann, H. (1986) A large chloroplast thioredoxin f found in green algae. Z. Naturforsch. 41c, 275–283
Laurent, T.C., Moore, E.C., Reichard, P. (1964) Enzymatic synthesis of deoxyribonucleotides. J. Biol. Chem. 239, 3436–3444
Maeda, K., Tsugita, A., Dalzoppo, D., Vilbois, F., Schürmann, P. (1986) Further characterization and amino acid sequence of m-type thioredoxins from spinach chloroplasts. Eur. J. Biochem. 154, 197–203
Marcus, F., Harrsch, P.B., Moberly, L., Edelstein, I., Latshaw, S.P. (1987) Spinach chloroplast fructose-1,6-bisphosphatase. Identification of the subtilisin-sensitive region and of conserved histidines. Biochemistry 26, 7029–7035
Padilla Peña, C.A. (1990) Sistemas tiorredoxina y glutarredoxina de mamíferos: Purificación y localizatión inmunocitoquímica en hipófisis. Ph. D. thesis, Universidad de Córdoba, Spain
Pajot, P. (1976) Fluorescence of proteins in 6-M guanidine hydrochloride. A method for the quantitative determination of tryptophan. Eur. J. Biochem. 63, 263–269
Plá, A., Chueca, A., López Gorgé, J. (1981) A new procedure for the purification of spinach leaf photosynthetic fructose-1,6-bisphosphatase by affinity chromatography on mercaptoethylamine-Sepharose. Photosynth. Res. 2, 291–296
Plá, A., López Gorgé, J. (1981) Thioredoxin/fructose-1,6-bisphosphatase affinity in the enzyme activation by the ferredoxinthioredoxin system. Biochim. Biophys. Acta 636, 113–118
Reutimann, H., Straub, B., Luisi, P.L., Holmgren, A. (1981) A conformational study of thioredoxin and its tryptic fragments. J. Biol. Chem. 256, 6796–6803
Rodríguez Andrés, A., Lázaro, J.J., Chueca, A., Hermoso, R., López Gorgé, J. (1987) Binding of photosynthetic fructose-1,6-bisphosphatase to chloroplast membranes. Plant Sci. 52, 41–48
Rodríguez Andrés, A., Lázaro, J.J., Chueca, A., Hermoso, R., López Gorgé, J. (1990) Effect of alcohols on the association of photosynthetic fructose-1,6-bisphosphatase to thylakoid membranes. Physiol. Plant. 78, 409–413
Sahrawy, M., Chueca, A., Hermoso, R., Lázaro, J.J., López Gorgé, J. (1990) In-vivo and in-vitro synthesis of photosynthetic fructose-1,6-bisphosphatase from pea (Pisum sativum L.). Planta 182, 319–324
Schmidt, A., Christen, U. (1978) A factor dependent sulfotransferase specific for 3′-phosphoadenosine-5′-phosphosulfate (PAPS) in the cyanobacterium Synechococcus 6301. Planta 140, 234–244
Schürmann, P., Maeda, K., Tsugita, A. (1981) Isomers in thioredoxins of spinach chloroplasts. Eur. J. Biochem. 116, 37–45
Soulié, J.M., Buc, J., Meunier, J.C., Pradel, J., Ricard, J. (1981) Molecular properties of chloroplastic thioredoxin f and the photoregulation of the activity of fructose 1,6-bisphosphatase. Eur. J. Biochem. 119, 497–502
Soulié, J.M., Buc, J., Rivière, M., Ricard, J. (1985) Equilibrium binding of thioredoxin fB to chloroplastic fructose bisphosphatase. Evidence for a thioredoxin site distinct from the active site. Eur. J. Biochem. 152, 565–568
Soulié, J.M., Rivière, M., Buc, J., Gontero, B., Ricard, J. (1987) Kinetics of the modulation of chloroplastic fructose-1,6-bisphosphatase activity by thioredoxin fb. Eur. J. Biochem. 162, 271–274
Whittaker, M.M., Gleason, F.K. (1984) Isolation and characterization of thioredoxin f from the filamentous cyanobacterium, Anabaena sp. 7119. J. Biol. Chem. 259, 14088–14093
Wolosiuk, R.A., Buchanan, B.B., Crawford, N.A. (1977) Regulation of NADP-malate dehydrogenase by the light-actuated ferredoxin/thioredoxin system of chloroplasts. FEBS Lett. 81, 253–258
Author information
Authors and Affiliations
Additional information
The authors are grateful to Mrs. Francisca Castro and Mr. Narciso Algaba for skilful technical assistance. This work was supported by grant PB87-0431 of Dirección General de Investigación Cientifica y Técnica (DGICYT, Spain).
Rights and permissions
About this article
Cite this article
Prado, F.E., Lázaro, J.J., Hermoso, R. et al. Purification and properties of pea (Pisum sativum L.) thioredoxin f, a plant thioredoxin with unique features in the activation of chloroplast fructose-1,6-bisphosphatase. Planta 188, 345–353 (1992). https://doi.org/10.1007/BF00192801
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00192801