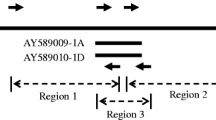
Abstract
A cDNA encoding the chloroplast/mitochondrial form of glutathione reductase (GR; EC 1.6.4.2) from pea (Pisum sativum L.) was used to map a single GR locus, named GOR1. In two domesticated genotypes of pea (cv. Birte and JI 399) it is likely that the GOR1 locus contains a single gene. However, in a semi-domesticated land race of pea (JI 281) two distinct but closely related sets of GR gene sequences were detected at the GOR1 locus. The extra GR sequences in JI 281 represent either a second intact gene or a partial or pseudogene copy. A GR gene was cloned from cv. Birte, sequenced and its structure analysed. No feature of the transcription or structure of the gene suggested a mechanism for generating any more than one form of GR. From these data plus previously published biochemical evidence we suggest that a second, distinct gene encoding for the cytosolic form of GR should be present in peas. The GOR1-encoded GR mRNA can be detected in all main organs of the plant and no alternative spliced species was present which could perhaps account for the generation of multiple isoforms of GR. The mismatch between the number of charge-separable isoforms in pea and the proposed number of genes suggests that different GR isoforms arise by some form of post-translational modification.
Similar content being viewed by others
Abbreviations
- GR:
-
glutathione reductase
- PCR:
-
polymerase chain reaction
- RACE:
-
rapid amplification of cDNA ends
- RFLP:
-
restriction fragment length polymorphism
References
Alscher RG (1989) Biosynthesis and antioxidant function of glutathione in plants. Physiol Plant 77: 457–464
Alting-Mees MA, Short JM (1989) pBluescript II, Gene mapping vectors. Nucleic Acids Res 17: 9494
Anderson JV, Hess JL, Chevone BI (1990) Purification, characterization, and immunological properties for two isoforms of glutathione reductase from eastern white pine needles. Plant Physiol 94: 1402–1409
Anderson MD, Prasad TK, Stewart CR (1995) Changes in isozyme profiles of catalase, peroxidase, and glutathione reductase during acclimation to chilling in mesocotyls of maize seedlings. Plant Physiol 109: 1247–1257
Asada K (1994) Production and action of active oxygen species in photosynthetic tissues. In: Foyer CH, Mullineaux PM (eds) Causes of photooxidative stress and amelioration of defense systems in plants. CRC Press, Boca Raton, pp 77–104
Benfey PN, Chua N-H (1990) Expression modules within the CaMV 35S promoter. Science 250: 958–966
Bielawski W, Joy KN (1986) Properties of glutathione reductase from chloroplasts and roots of pea. Phytochemistry 25: 2261–2265
Broadbent P, Creissen GP, Kular B, Wellburn AR, Mullineaux PM (1995) Oxidative stress responses in transgenic tobacco containing altered levels of glutathione reductase activity. Plant J 8: 247–255
Castresana C, Garcia-Luque I, Alonso E, Malik VS, Cashmore AR (1988) Both positive and negative regulatory elements mediate expression of a photoregulated CAB gene from Nicotiana plumbaginifolia. EMBO J 7: 1929–1936
Connell JP, Mullet JE (1986) Pea chloroplast glutathione reductase: Purification and characterization. Plant Physiol 82: 351–356
Creissen G, Edwards EA, Enard C, Wellburn A, Mullineaux P (1992) Molecular characterisation of glutathione reductase cDNAs from peas. Plant J 2: 29–131
Creissen G, Edwards EA, Mullineaux P (1994) Glutathione reductase and ascorbate peroxidase. In: Foyer CH, Mullineaux PM (eds) Causes of photooxidative stress and amelioration of defense systems in plants. CRC Press, Boca Raton, pp 343–364
Creissen G, Reynolds H, Xue Y, Mullineaux P (1995) Simultaneous targeting of pea glutathione reductase and of a bacterial fusion protein to chloroplasts and mitochondria in transgenic tobacco. Plant J 8: 167–175
Creissen GP, Mullineaux PM (1995) Cloning and characterisation of glutathione reductase cDNAs and identification of two genes encoding the tobacco enzyme. Planta 197: 422–425
Devereux J, Haeberi P, Smithies O (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 12: 387–395
Drumm-Herrel H, Gerhausser U, Mohr H (1989) Differential regulation by phytochrome of the appearance of plastidic and cytoplasmic isoforms of glutathione reductase in mustard (Sinapis alba L.)cotyledons. Planta 178: 103–109
Edwards EA, Rawsthorne S, Mullineaux PM (1990) Subcellular distribution of multiple forms of glutathione reductase in leaves of pea (Pisum sativum L.). Planta 180: 278–284
Edwards EA, Enard C, Creissen GP, Mullineaux PM (1994) Synthesis and properties of glutathione reductase in stressed peas. Planta 192: 137–143
Ellis THN, Turner L, Hellens RP, Lee D, Harker CL, Enard C, Domoney C, Davies DR (1992) Linkage maps in pea. Genetics 130: 649–663
Feinberg AP, Vogelstein B (1983) A technique for radiolabelling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132: 6–13
Forster C, Arthur E, Crespi S, Hobbs SLA, Mullineaux P, Casey R (1994) Isolation of a pea (Pisum sativum) seed lipoxygenase promoter by inverse polymerase chain reaction and characterization of its expression in transgenic tobacco. Plant Mol Biol 26: 235–248
Foyer CH and Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbate metabolism. Planta 133: 21–25
Foyer C, Lelandais M, Galap C, Kunert KJ (1991) Effects of elevated cytosolic glutathione reductase activity on the cellular glutathione pool and photosynthesis in leaves under normal and stress conditions. Plant Physiol 97: 863–872
Frohman MA (1990) RACE: Rapid amplification of cDNA ends. In: Innis MA, Gelfland DH, Sninsky JJ, White TJ (eds) PCR Protocols: A guide to methods and applications, pp 28–38. Academic Press, San Diego
Ghosh D (1990) A relational database of transcription factors. Nucleic Acids Res 18: 1749–1756
Glackin CA, Grula JW (1990) Organ-specific transcripts of different size and abundance derive from the same pyruvate, orthophosphate dikinase gene in maize. Proc Natl Acad Sci USA 87: 3004–3008
Grotewald E, Athama P, Peterson T (1991) Alternatively spliced products of the maize P gene encode proteins with homology to the DNA-binding domain of myb-like transcription factors. Proc Natl Acad Sci USA 88: 4587–4591
Guerineau F, Brooks L, Mullineaux P (1991) Effect of deletions in the cauliflower mosaic virus polyadenylation sequence on the choice of the polyadenylation sites in tobacco protoplasts. Mol Gen Genet 226: 141–144
Guy CL, Carter JV (1984) Characterization of partially purified glutathione reductase from cold-hardened and non-hardened spinach leaf tissue. Cryobiology 21: 454–464
Halliwell B, Foyer CH (1978) Properties and physiological function f a glutathione reductase purified from spinach leaves by affinity chromatography. Planta 139: 9–17
Hausladen A, Alscher RG (1994) Purification and characterization of glutathione reductase isozymes specific for the state of cold hardiness of red spruce. Plant Physiol 105: 205–213
Herouart D, Van Montagu M, Inzé D (1993) Redox-activated expression of the cytosolic copper/zinc superoxide dismutase gene in Nicotiana. Proc Natl Acad Sci USA 90: 3108–3112
Innis MA, Gelfland DH (1990) Optimization of PCRs. In: Innis MA, Gelfland DH, Sninsky JJ, White TJ (eds) PCR Protocols: A guide to methods and applications, pp 3–12. Academic Press, San Diego
Kalt-Torres W, Burke JJ, Anderson JM (1984) Chloroplast glutathione reductase: Purification and properties. Physiol Plant 61: 271–278
Klapheck S, Zimmer I, Cosse H (1990) Scavenging of hydrogen peroxide in the endosperm of Ricinus communis by ascorbate peroxidase. Plant Cell Physiol 31: 1005–1013
Knox M, Forster C, Domoney C, Casey R (1994) Structure of the Pisum sativum seed lipoxygenase gene lox1, Ps, 3. Biochim Biophys Acta 1214: 341–343
Kubo A, Sano T, Saji H, Tanaka K, Kondo N, Tanaka K (1993) Primary structure and properties of glutathione reductase from Arabidopsis thaliana. Plant Cell Physiol 34: 1259–1266
Kunert KJ, Cresswell CF, Schmidt A, Mullineaux PM, Foyer CH (1990) Variations in the activity of glutathione reductase and the cellular glutathione content in relation to sensitivity to methylviologen in Escherichia coli. Arch Biochem Biophys 282: 233–238
Madamanchi NR, Anderson JV, Alscher RG, Cramer CL, Hess JL (1992) Purification of multiple forms of glutathione reductase from pea (Pisum sativum L.) seedlings and enzyme levels in ozone-fumigated pea leaves. Plant Physiol 100: 138–145
Mahan JR, Burke JJ (1987) Purification and characterization of glutathione reductase from corn mesophyll chloroplasts. Physiol Plant 71: 352–358
Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52: 711–760
Melton DA, Krieg PA, Rebagliati MR, Maniatis T, Zinn K, Green MR (1984) Efficient in vitro synthesis of biologically active RNA and RNA hybridisation probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res 12: 7035–7056
Mittler R, Zilinskas BA (1992) Molecular cloning and characterization of a gene encoding pea cytosolic ascorbate peroxidase. J Biol Chem 267: 21802–21807
Mullineaux PM, Rigden JE, Dry IB, Krake LR, Rezaian MA (1993) Mapping of the polycistronic RNAs of tomato leaf curl gemini virus. Virology 193: 414–423
Polle A, Rennenberg H (1994) Photooxidative stress in trees. In: Foyer CH, Mullineaux PM (eds) Causes of photooxidative stress and amelioration of defense systems in plants. CRC Press, Boca Raton, pp 199–218
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning. A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467
Scruton NS, Berry A, Perham RN (1990) Redesign of the coenzyme specificity of a dehydrogenase by protein engineering. Nature 343: 38–43
Sies H (1985) Oxidative stress. Introductory remarks. In: Sies H (ed) Oxidative stress. Academic Press, London, pp 1–8
Simpson CG, Leader DJ, Brown JWS, Franklin T (1993) Characteristics of plant pre-mRNA introns and transposable elements. In: Croy RRD (ed) Plant molecular biology LABFAX. Bios Scientific Publishers, Oxford, pp 183–252
Smith IK, Polle A, Rennenberg H (1990) Glutathione. In: Alscher RG, Cumming JR (eds) Stress responses in plant adaptation and acclimation mechanisms. Wiley-Liss, New York, pp 201–215
Staden R (1984) A computer program to enter DNA gel reading data into a computer. Nucleic Acids Res 12: 499–503
Steffens JC (1990) The heavy metal-binding peptides of plants. Annu Rev Physiol Plant Mol Biol 41: 553–575
Strid A (1993) Alteration in expression of defence genes in Pisum sativum after exposure to supplementary ultraviolet-B radiation. Plant Cell Physiol 34: 949–953
Struhl K (1985) Naturally occurring poly(dA-dT) sequences are upstream promoter elements for constitutive transcription in yeast. Proc Natl Acad Sci USA 82: 8419–8423
Tanaka K, Saji H, Kondo N (1988) Immunological properties of spinach glutathione reductase and inductive biosynthesis of the enzyme with ozone. Plant Cell Physiol 29: 637–642
Tang X, Webb MA (1994) Soybean root nodule cDNA encoding glutathione reductase. Plant Physiol 104: 1081–1082
Timmermann KP (1989) Molecular characterization of corn glutathione S-transferase isozymes involved in herbicide detoxification. Physiol Plant 77: 465–471
Voss H, Wiemann U, Wirkner U, Schwager C, Zimmermann J, Stegemann J, Erfle H, Hewitt NA, Rupp T, Ansorge W (1992) Automated DNA sequencing system resolving 1000 bases with fluorescein-15-dATP as internal label. Methods Mol Cell Biol 3: 30–34
Wingate VPM, Lawton MA, Lamb CJ (1988) Glutathione causes a massive and selective induction of plant defense genes. Plant Physiol 87: 206–210
Wingsle G (1989) Purification and characterization of glutathione reductase from Scots pine needles. Physiol Plant 76: 24–30
Wingsle G, Karpinski S (1995) Differential redox regulation by glutathione of glutathione reductase and CuZn-superoxide dismutase gene expression in Pinus sylvestris L. needles. Planta 198: 151–157
Woolston CJ, Barker R, Gunn H, Boulton MI, Mullineaux PM (1988) Agroinfection and nucleotide sequence of cloned wheat dwarf virus DNA. Plant Mol Biol 11: 35–43
Yanisch-Perron C, Vieira J, Messing J (1985) Improved M13 phage cloning vectors and host strains. Gene 33: 103–119
Author information
Authors and Affiliations
Corresponding author
Additional information
The pea glutathione reductase gene sequences will appear in the EMBL/Genbank databases with the accession number X90996
The authors are grateful to Dr. S. Karpinski for critical reading of the manuscript. This work was supported by a grant-in-aid from the Biotechnology and Biological Sciences Research Council (BBSRC) to the John Innes Centre. C.E. is grateful for a fellowship from the European Molecular Biology Organisation. We acknowledge the support of the BBSRC Global Environmental Change Programme and the support of the Commission of the European Union Framework 3 Agro-Industrial Programme (contract no. AIR-CT92-0205).
Rights and permissions
About this article
Cite this article
Mullineaux, P., Enard, C., Hellens, R. et al. Characterisation of a glutathione reductase gene and its genetic locus from pea (Pisum sativum L.). Planta 200, 186–194 (1996). https://doi.org/10.1007/BF00208308
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00208308