Abstract
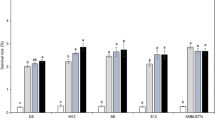
A method based on the survival of yeast cells subjected to an ethanol or heat shock was utilized to compare the stress resistance of free and carrageenan-immobilized yeast cells. Results demonstrated a significant increase of yeast survival against ethanol for immobilized cells as compared to free cells, while no marked difference in heat resistance was observed. When entrapped cells were released by mechanical disruption of the gel beads and submitted to the same ethanol stress, they exhibited a lower survival rate than entrapped cells, but a similar or slightly higher survival rate than free cells. The incidence of ethanol- or heat-induced respiratory-deficient mutants of entrapped cells was equivalent to that of control or non-stressed cells (1.3 ± 0.5%) whereas ethanol- and heat-shocked free and released cells exhibited between 4.4% and 10.9% average incidence of respiration-deficient mutants. It was concluded that the carrageenan gel matrix provided a protection against ethanol, and that entrapped cells returned to normal physiological behaviour as soon as they were released. The cell growth rate was a significant factor in the resistance of yeast to high ethanol concentrations. The optimum conditions to obtain reliable and reproducible results involved the use of slow-growing cells after exhaustion of the sugar substrate.
Similar content being viewed by others
References
Aquilera A, Benitez T (1989) Synergistic effects of ethanol and temperature on yeast mitochondria. Curr Microbiol 18:179–188
Arnaud J-P, Lacroix C (1991) Diffusion of lactose in κ-carrageenan/locust bean gum gel beads with or without entrapped lactic acid bacteria. Biotechnol Bioeng 38:1041–1049
Audet P, Lacroix C (1989) Two phase dispersion process for the production of biopolymer gel beads: effect of various parameters on bead size and their distribution. Proc Biochem 24:217–226
Briggs DE, Hough JS, Stevens R, Young TW (1981) Malting and brewing science. Chapman & Hall, London
Cabeca-Silva C, Madeira-Lopes A, Uden N van (1982) Temperature relation of ethanol-enhanced petite mutation in Saccharomyces cerevisiae. FEMS Microbiol Lett 15:149–151
Casey GP, Ingledew WM (1986) Ethanol tolerance in yeasts. Crit Rev Microbiol 13:218–280
Chen C, Dale MC, Okos MR (1990) The long-term effects of ethanol on immobilized cell reactor performance using K. fragilis. Biotechnol Bioeng 36:975–982
D'Amore T, Panchal CJ, Stewart GG (1987) The effect of osmotic pressure on the intracellular accumulation of ethanol of Saccharomyces cerevisiae during fermentation in wort. J Inst Brew 93:472–476
D'Amore T, Panchal CJ, Russell I, Stewart GG (1990) A study of ethanol tolerance in yeast. Crit Rev Biotechnol 9:287–304
Dror Y, Cohen O, Freeman A (1988) Stabilization effect on immobilized yeast: effect of gel composition on tolerance to water miscible solvents. Enzyme Microb Technol 10:273–279
Folch J, Lees M, Sloane-Stanley, GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Galazzo JL, Bailey JE (1990) Growing Saccharomyces cerevisiae in calcium-alginate beads indices cell alterations which accelerate glucose conversion to ethanol. Biotechnol Bioeng 36:417–426
Hilge-Rotmann B, Rehm HJ (1991) Relationship between fermentation capability and fatty acid composition of free and immobilized Saccharomyces cerevisiae. Appl Microbiol Biotechnol 34:502–508
Holcberg IB, Margalith P (1981) Alcoholic fermentation by immobilized yeast at high sugar concentration. Eur J Appl Microbiol Biotechnol 13:133–140
Jirkü V (1991) The effect of covalent immobilization on ethanol-induced leakage in Saccharomyces cerevisiae. Acta Biotechnol 11:77–80
Keweloh H, Heipieper HJ, Rehm HJ (1989) Protection of bacteria against toxicity of phenol by immobilization in calcium alginate. Appl Microbiol Biotechnol 31:383–389
Letters R (1966) Phospholipids in yeast. II. Extraction, isolation and characterization of yeast phospholipids. Biochim Biophys Acta 16:489–494
Lewis JG, Northcott CJ, Learmonth RP, Attfield PV, Watson K (1993) The need for consistent nomenclature and assessment of growth phases in diauxic cultures of Saccharomyces cerevisiae. J Gen Microbiol 139:835–839
Masschelein CA, Carlier A, Ramos-Jeunehomme C, Abe I (1985) The effect of immobilization on yeast physiology and beer quality in continuous and discontinuous systems. Proceedings of the European Brewing Convention, Helsinki. IRL Press, Oxford, England, pp 339–346
Norton S, Lacroix C, Vuillemard JC (1993) Effect of pH on the morphology of Lactobacillus helveticus in free-cell batch and immobilized-cell continuous fermentation. Food Biotechnol 7:235–251
Ogur M, St John R (1956) A differential and diagnostic plating method for population studies of respiration deficiency in yeast. J Bacteriol 72:500–504
Richter K, Rühlemann I, Becker U, Berger R (1989) A comparison between the fermentative activities of free and Ca-alginate-entrapped cells of Saccharomyces cerevisiae. Acta Biotechnol 9:123–129
Sherman F (1959) The effects of elevated temperatures on yeast. II. Induction of respiratory-deficient mutants. J Cell Comp Physiol 54:37–52
Silhankova L, Savel J, Mostek J (1970) Respiratory deficient mutants of bottom brewer's yeast. I. Frequencies and types of mutant in various strains. J Inst Brew 76:280–288
Watson K (1987) Temperature relations. In: Rose AH, Harrison, JS (eds) The yeasts, 2nd edn. vol 2. Academic Press, London, pp 41–71
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Norton, S., Watson, K. & D'Amore, T. Ethanol tolerance of immobilized brewers' yeast cells. Appl Microbiol Biotechnol 43, 18–24 (1995). https://doi.org/10.1007/BF00170616
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00170616