Abstract
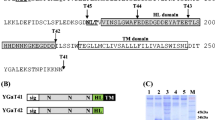
As the first step for production of rat apolipoprotein E (rApoE) in Saccharomyces cerevisiae, the rApoE cDNA was cloned and its nucleotide sequence was determined. When the intact rApoE gene including the presequence-encoding region was expressed under the control of the yeast GAL7 promoter, no protein immunoreactive with anti-rApoE antibody was detected either in the culture medium or inside the cells. For the purpose of the extracellular production of rApoE, three fusion genes were constructed in which the mature rApoE-encoding sequence was connected after the pre, prepro, and whole regions of the gene encoding a fungal aspartic proteinase, Mucor pusillus rennin (MPP), since MPP is efficiently secreted from recombinant S. cerevisiae containing the MPP gene. When these three fusion genes were expressed under the control of the GAL7 promoter, only one, encoding the mature rApoE connected to the whole MPP sequence, directed efficient secretion of the fused protein. The maximum yield of the fused protein secreted into the medium reached 11.8 mg/l and the calculated rApoE part was 5.3 mg in the fused protein. The excreted fusion protein was glycosylated at the original two sites in the MPP part. The fused protein was gradually degraded in the medium probably by proteases of the host cell, because no such degradation occured in a yeast pep4mutant strain.
Similar content being viewed by others
References
Aikawa J, Yamashita T, Nishiyama M, Horinouchi S, Beppu T (1990) Effect of glycosylation on the secretion and enzyme activity of Mucor rennin, an aspartic proteinase of Mucor pusillus, produced by recombinant yeast. J Biol Chem 265:13955–13959
Ammerer G, Hunter CP, Rothman JH, Saari GC, Valls LA, Stevens TH (1986) The PEP4 gene of Saccharomyces cerevisiae encodes protease A, a vacuolar enzyme required for processing of vacuolar precursors. Mol Cell Biol 6:2490–2499
Asagoe Y, Yasukawa K, Saito T, Maruo N, Miyata K, Kono T, Miyake T, Kato T, Kakidani H, Mitani M (1988) Human B-cell stimulatory factor-2 expressed in Escherichia coli. Bio/Technology 6:806–809
Bitter GA, Chen KK, Banks AR, Lai PH (1984) Secretion of foreign proteins from Saccharomyces cerevisiae directed by α-factor gene fusions. Proc Natl Acad Sci USA 81:5330–5334
Etoh Y, Shoun H, Beppu T, Arima K (1979) Milk-clotting enzyme from microorganisms. Part XII. Physicochemical and immunochemical studies on similarities of the acid proteases, Mucor pusillus rennin and Mucor miehei rennin. Agric Biol Chem 43:209–215
Fung W-P, Howlett GJ, Schreiber G (1986) Structure and expression of the rat apolipoprotein E gene. J Biol Chem 261:13777–13783
Hemmings BA, Zubenko GS, Hasilik A, Jones EW (1981) Mutant defective in processing of an enzyme located in the lysozyme-like vacuole of Saccharomyces cerevisiae. Proc Natl Acad Sci USA 78:435–439
Hiramatsu R, Aikawa J, Horinouchi S, Beppu T (1989) Secretion by yeast of the zymogen form of Mucor rennin, an aspartic proteinase of Mucor pusillus, and its conversion to the mature form. J Biol Chem 264:16862–16866
Hiramatsu R, Yamashita T, Aikawa J, Horinouchi S, Beppu T (1990) The prepro-peptide of Mucor rennin directs the secretion of human growth hormone by Saccharomyces cerevisiae. Appl Environ Microbiol 56:2125–2132
Hiramatsu R, Horinouchi S, Beppu T (1991) Isolation and characterization of human pro-urokinase and its mutants accumulated within the yeast secretory pathway. Gene 99:235–241
Ho SB, Hunt HD, Horton RM, Pullen JK, Pease LR (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51–59
Itoh H, Fukuda Y, Murata K, Kimura A (1983) Transformation of intact yeast cells treated with alkali cations. J Bacteriol 153:163–168
Mahley RW (1988) Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 240:622–630
McLean JW, Fukasawa C, Taylor JM (1983) Rat apolipoprotein E mRNA. J Biol Chem 258:8993–9000
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Smith RA, Duncan MJ, Moir DT (1985) Heterologous protein secretion from yeast. Science 229:1219–1224
Sturley SL, Culbertson MR, Attie AD (1991) Secretion and lipid association of human apolipoprotein E in Saccharomyces cerevisiae requires a host mutation in sterol esterification and uptake. J Biol Chem 266:16273–16276
Tajima M, Nogi Y, Fukasawa T (1985) Primaty structure of the Saccharomyces cerevisiae GAL7 gene. Yeast 1:67–77
Tarentino AL, Plummer THJ, Maley F (1974) The release of intact oligosaccharides from specific glycoproteins by endo-β-N-acetylglucosaminidase H J Biol Chem 249:818–824
Tonouchi N, Shoun H, Uozumi T, Beppu T (1986) Cloning and sequencing of a gene for Mucor rennin, an aspartic protease from Mucor pusillus. Nucleic Acids Res 14:7557–7568
Woolford CA, Daniels LB, Park FJ, Jones EW, van Arsdell JN, Innis MA (1986) The PEP4 gene encodes an aspartyl protease implicated in the posttranslational regulation of Saccharomyces cerevisiae vaculor hydrolases. Mol Cell Biol 6:2500–2510
Yamashita T, Tonouchi N, Uozumi T, Beppu T (1987) Secretion of Mucor rennin, a fungal aspartc protease of Mucor pusillus, by recombinant yeast cells. Mol Gen Genet 210:462–467
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nomura, N., Yamada, H., Matsubara, N. et al. Secretion by Saccharomyces cerevisiae of rat apolipoprotein E as a fusion to Mucor rennin. Appl Microbiol Biotechnol 42, 865–870 (1995). https://doi.org/10.1007/BF00191183
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00191183