Summary
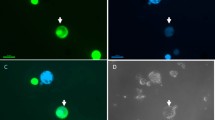
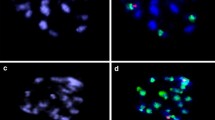
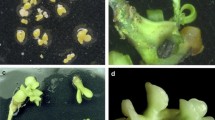
For our program on the transfer of cytoplasmic male sterility (CMS) by cybridization inBeta vulgaris L. (sugar beet), we have developed a procedure for the isolation and culture of mesophyll protoplasts of sugar beet followed by shoot regeneration. A prerequisite proved to be the presence in the media of n-propylgallate (nPG), a lipoxygenase inhibitor. Sustained divisions were found in all accessions that were tested. Plating efficiencies and regeneration ability varied greatly from one experiment to the other and appeared to be accession-dependent. Shoots could be easily transferred to soil. A majority of the regenerants (72%) retained the diploid chromosome number. Somaclonar variation in phenotype was low (4.9%). Mitochondrial DNA probes, capable of discriminating different cytoplasms ofBeta spp. showed no rearrangements due to the protoplast and in vitro culture phase, indicating that these probes can be used to identify cybrids after asymmetric fusions. The data presented here open up possibilities for genetic engineering using protoplasts in one of the world's most important arable crops.
Similar content being viewed by others
References
Akagi H, Sakamoto M, Negishi T, Fujimura T (1989) Construction of rice cybrid plants. Mol Gen Genet 215:501–506
Barsby TL, Yarrow SA, Kemble RJ, Grant I (1987) The transfer of cytoplasmic male sterility to winter-type oilseed rape (Brassica napus L.) by protoplast fusion. Plant Sci 53:243–248
Bhat SR, Ford-Lloyd BV, Callow JA (1986) Isolation and culture of mesophyll protoplasts of garden, fodder, and sugar beets using a nurse culture system: callus formation and organogenesis. J Plant Physiol 124:419–423
Boutin V, Pannenbecker G, Ecke W, Schewe G, Samitou-Laprade P, Jean R, Vernet Ph (1987) Cytoplasmic male sterility and nuclear restorer genes in a natural population ofBeta maritima: genetical and molecular aspects. Theor Appl Genet 73:625–629
Brears T, Curtis GJ, Lonsdale DM (1989) A specific rearrangement of mitochondrial DNA induced by tissue culture. Theor Appl Genet 77:620–624
De Greef W, Jacobs M (1979) In vitro culture of the sugar beet: description of a cell line with high regeneration capacity. Plant Sci Lett 17:55–61
Dellaporta SJ (1983) A plant DNA minipreparation version II. Plant Mol Biol Rep 1:19–21
Detrez C, Sangwan RS, Sangwan-Norreel BS (1989) Phenotypic and karyotypic status ofBeta vulgaris plants regenerated from direct organogenesis in petiole culture. Theor Appl Genet 77:462–468
Frearson EM, Power JB, Cocking EC (1973) The isolation, culture, and regeneration ofPetunia leaf protoplasts. Dev Biol 33:130–137
Freytag AH, Anand SC, Rao-Ardelli AP, Owens LD (1988) An improved medium for adventitious shoot formation and callus induction inBeta vulgaris L. in vitro. Plant Cell Rep 7:30–34
Hall RD, Krens FA (1988) The production and electrofusion ofBeta cytoplasts. In: Puite KJ, Dons JJM, Huizing HJ, Kool AJ, Koornneef M, Krens FA (eds) Progress in plant protoplast research. Kluwer, Dordrecht, pp 263–264
Halldén C, Bryngelsson T, Bosemark NO (1988) Two new types of cytoplasmic male sterility found in wildBeta beets. Theor Appl Genet 75:561–568
Hooker AL, Smith DR, Lim SM, Beckett JB (1970) Reaction of corn seedlings with male sterile cytoplasm toHelminthosporium maydis. Plant Dis Rep 54:708–712
Izhar S, Schlicter M, Swartzberg D (1983) Sorting out of cytoplasmic elements in somatic hybrids ofPetunia and the prevalence of the heteroplasmon through several meiotic cycles. Mol Gen Genet 190:468–474
Kafatos FC, Jones CW, Efstratiadis A (1979) Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res 7:1541–1552
Kao KN, Michayluk MK (1975) Nutritional requirements for growth ofVinca hajastana cells and protoplasts at a very low density in liquid media. Planta 126:105–110
Krens FA, Jamar D (1989) The role of expiant source and culture conditions on callus induction and shoot regeneration in sugar beet (Beta vulgaris L.). J Plant Physiol 134: 651–655
Krens FA, Molendijk I., Wullems GJ, Schilperoort RA (1985) The role of bacterial attachment in the transformation of cell-wall regenerating tobacco protoplasts byAgrobacterium tumefaciens. Planta 166:300–308
Leaver CJ, Gray MW (1982) Mitochondrial genome organization and expression in higher plants. Annu Rev Plant Physiol 33:373–402
Lee-Stadelmann O, Chung I, Stadelmann EJ (1985) Plasmolysis ofGlycine max mesophyll cells: the use of octylguanidine and its implications in protoplast isolation. Plant Sci 38:1–7
Lindsey K, Jones MGK (1987) The permeability of electroporated cells and protoplasts of sugar beet. Planta 172:346–355
Menczel I., Morgan A, Brown S, Maliga P (1987) Fusion-mediated combination of Ogura-type cytoplasmic male sterility withBrassica napus plastids using X-irradiated CMS protoplasts. Plant Cell Rep 6:98–101
Mikami T, Kishima Y, Sugiura M, Kinoshita T (1985) Organelle genome diversity in sugar beet with normal and different sources of male sterile cytoplasms. Theor Appl Genet 71:166–171
Miller RJ, Koeppe DE (1971) Southern corn leaf blight: susceptible and resistant mitochondria. Science 173:67–69
Owen FV (1945) Cytoplasmically inherited male sterility in sugar beet. J Agric Res 71:423–440
Saleem M, Cutler AJ (1987) Stabilizing corn leaf protoplasts with n-propylgallate. J Plant Physiol 128:479–484
Samitou-Laprade P, Rouwendal GJA, Cugnen J, Krens FA, Michaelis G (1990) CMS inBeta ssp.: mitochondrial variation in populations as revealed by nonradioactive Southern blot analysis (in press)
Sree-Ramulu K, Dijkhuis P, Roest S (1983) Phenotypic variation and ploidy level of plants regenerated from protoplasts of tetraploid potato (Solanum tuberosum L. cv “Bintje”). Theor Appl Genet 65:329–338
Tanno-Suenaga I., Ichikawa H, Imamura J (1988) Transfer of the CMS trait inDaucus carota L. by donor-recipient protoplast fusion. Theor Appl Genet 76:855–860
Yang ZQ, Shikanai T, Mori K, Yarnada Y (1989) Plant regeneration from cytoplasmic hybrids of rice (Oryza sativa L.). Theor Appl Genet 77:305–310
Zelcer A, Aviv D, Galun E (1978) Interspecific transfer of cytoplasmic male sterility by fusion between protoplasts of normalNicotiana sylvestris and X-ray irradiated protoplasts of male-sterileN. tabacum. Z Pflanzenphysiol 90:397–407
Author information
Authors and Affiliations
Additional information
Communicated by Yu. Gleba
Rights and permissions
About this article
Cite this article
Krens, F.A., Jamar, D., Rouwendal, G.J.A. et al. Transfer of cytoplasm from newBeta CMS sources to sugar beet by asymmetric fusion. Theoret. Appl. Genetics 79, 390–396 (1990). https://doi.org/10.1007/BF01186084
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01186084