Abstract
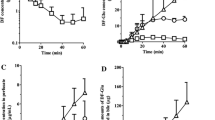
A multiple cannulated rat model was utilized to investigate the relative contribution of the gut and liver as sites of first-pass metabolism of orally administered U-54494 A, an anticonvulsant drug candidate. Each rat received a dose of U-54494 A by oral, intraportal, and intravenous routes on three separate occasions. Intraportal and intravenous doses were administered through chronic cannulas surgically implanted in the portal vein and superior vena cava, respectively. Blood samples were collected over a 6-hr period from the superior vena cava cannula. The mean (n = 3) bioavailability of orally dosed U-54494A was 4.5 ± 1.1%, while that dosed intraportally was 19.1 ± 3.0%. The relative contribution of the gut and liver as sites of first-pass extraction and/or metabolism of orally administered drug was 69.9 ± 14.0% and 24.5 ± 12.2%, respectively. Approximately 35 to 40% of the total plasma clearance was attributed to the liver. The plasma concentrations of the four known metabolites of U-54494A were apparently higher for the oral and intraportal routes compared to that after intravenous administration. This investigation confirms that the low oral bioavailability of U-54494A in the rat can be primarily attributed to both extensive intestinal and hepatic first-pass metabolism.
Similar content being viewed by others
REFERENCES
P. F. VonVoigtlander, E. D. Hall, M. C. Ochoa, R. A. Lewis, and H. J. Triezenberg. U-54494A: A unique anticonvulsant related to kappa opioid agonists. J. Pharmacol. Exp. Ther. 243:542–547 (1987).
W. Z. Zhong, and M. G. Williams. Quantitative determination of cis-3,4-dichloro-N-methyl-N-(2-(1-pyrrolidinyl)-cyclohexyl)-benzamide and three of its metabolites in plasma by high-performance liquid chromatography. J. Pharm. Sci. 82:1049–1053 (1993).
R. A. Morrison, D. E. Burkett, M. E. Arnold, C. J. D'Arienzo, and S. H. Weinstein. Sites of first-pass bioactivation (hydrolysis) of orally administered zofenopril calcium in dogs. Pharmaceutical Research. 8:370–375 (1991).
F. Chanoine, C. Grenot, P. Heidmann, and J. L. Junien. Pharmacokinetics of butixocort 21-propionate, budesonide, and beclomethasone dipropionate in the rat after intratracheal, intravenous, and oral treatments. Drug Metab. Dispos. 19:546–553 (1991).
D. Brewster, M. J. Humphrey, and M. A. McLeavy. The systemic bioavailability of buprenorphine by various routes of administration. J. Pharm. Pharmacol. 33:500–506 (1981).
M. K. Cassidy, and J. B. Houston. In vivo assessment of extrahepatic conjugative metabolism in first pass effects using the model compound phenol. J. Pharm. Pharmacol. 32:57–59 (1980).
W. D. Conway, S. M. Singhvi, M. Gibaldi, and R. N. Boyes. The effect of route of administration on the metabolic fate of terbutaline in the rat. Xenobiotica. 3:813–821 (1973).
K. Iwamoto and C. D. Klaassen. First-pass effect of morphine in rats. J. Pharmacol. Exp. Ther. 200(1):236–244 (1977).
K. F. Ilett, and C. T. Dollery, and D. S. Davies. Isoprenaline conjugation—a “true” first-pass effect” in the dog intestine. J. Pharm. Pharmacol. 32:362 (1980).
H. B. Waynforth. Experimental and Surgical Technique in the Rat. Academic Press, London. 1980, pp. 50–57 and 72–74.
D. M. Cocchetto, and T. D. Bjornsson. Methods for vascular access and collection of body fluids from the laboratory rat. J. Pharm. Sci. 72:465–492 (1983).
J. R. Weeks, and J. A. Jones. Routine direct measurement of arterial pressure in unanesthetized rats. Proc. Soc. Exp. Biol. Med. 104:646–648 (1960).
M. Gibaldi, and D. Perrier. Pharmacokinetics, Second Edition, Marcel Dekker, New York, 1982, pp. 5, 411.
K. B. Bischoff, R. L. Dedrick, D. S. Zaharko, and J. A. Longstreth. Methotrexate pharmacokinetics. J. Pharm. Sci. 60: 1128–1133 (1971).
H. Boxenbaum. Interspecies variation in liver weight, hepatic blood flow, and antipyrine intrinsic clearance: extrapolation of data to benzodiazepines and phenytoin. J. Pharmacokin. Biopharm. 8:165–176 (1980).
J. L. Gabrielsson, P. Johansson, U. Bondesson, and L. K. Paalzow. Analysis of methadone disposition in the pregnant rat by means of a physiological flow model. J. Pharmacokin. Biopharm. 13:355–372 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhong, WZ., Williams, M.G., Cook, K.J. et al. First-Pass Effect of cis-3,4-Dichloro-N-methyl-N-(2-(l-pyrrolidinyl)-cyclohexyl)-benzamide (U-54494) in Rats— A Model with Multiple Cannulas for Investigation of Gastrointestinal and Hepatic Metabolism. Pharm Res 11, 1524–1529 (1994). https://doi.org/10.1023/A:1018985015596
Issue Date:
DOI: https://doi.org/10.1023/A:1018985015596