Abstract
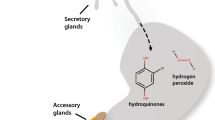
The abdominal defensive glands ofC. maxillosus secrete a mixture (70μg/beetle) of isoamyl alcohol (I), isoamyl acetate (II), iridodial (III), actinidine (IV), dihydronepetalactone (VE), and (E)-8-oxocitronellyl acetate (X). When disturbed, the beetle everts the glands and revolves the abdomen so as to wipe the glands against the offending agent. Fecal fluid is commonly emitted at the same time and may become added to the glandular material. Ants (Formica exsectoides) are effectively fended off by the beetle and were shown in bioassays (Monomorium destructor) to be repelled by the four major components of the secretion (II, III, X, VE); the principal component (VE) was the most active. Some anatomical features of the glands are described.
Similar content being viewed by others
References
Bellas, T.E., Brown, W.V., andMoore, B.P. 1974. The alkaloid actinidine and plausible precursors in defensive secretions of rove beetles.J. Insect Physiol. 20:277–280.
Bettini, S. (ed.). 1978. Arthropod Venoms. Handbook of Experimental Pharmacology, Vol.48. Springer-Verlag, Berlin.
Blum, M.S., Crewe, R.M., andPasteels, J.M. 1971. Defensive secretion ofLomechusa strumosa, a myrmecophilous beetle.Ann. Entomol. Soc. Am. 64:975–976.
Boch, R., Shearer, D.A., andStone, B.C. 1962. Identification ofiso-amyl acetate as an active compound in the sting pheromone of the honey bee.Nature 195:1018–1020.
Brand, J.M., Blum, M.S., Fales, H.M., andPasteels, J.M. 1973. The chemistry of the defensive secretion of the beetle,Drusilla canaliculata.J. Insect. Physiol. 19:369–382.
Clark, K., Fray, G.I., Jaeger, R.H., andRobinson, R. 1959. Synthesis ofd- andl-iso- iridomyrmecin and related compounds.Tetrahedron 6:217–224.
Eisner, T. 1960. Defense mechanisms of arthropods. II. The chemical and mechanical weapons of an earwig.Psyche 67:62–70.
Eisner, T. 1964. Catnip: Its raison d'être.Science 146:1318–1320.
Eisner, T. 1970. Chemical defense against predation in arthropods, pp. 157–217,in E. Sondheimer, and J.B. Simeone (eds.). Chemical Ecology. Academic Press, New York.
Eisner, T., andMeinwald, Y.C. 1965. Defensive secretion of a caterpillar (Papilio).Science 150:1733–1735.
Eisner, T., andMeinwald, J. 1966. Defensive secretion of arthropods.Science 153:1341–1350.
Eisner, T., Meinwald, J., Monro, A., andGhent, R. 1961. Defense mechanisms of arthropods—I. The composition and function of the spray of the whip-scorpion,Mastigoproctus giganteus (Lucas) (Arachnida, Pedipalpida).J. Insect Physiol. 6:272–298.
Eisner, T., Kluge, A.F., Carrel, J.E., andMeinwald, J. 1971. Defense of phalangid: liquid repellent administered by leg dabbing.Science 173:650–652.
Eisner, T., Hill, D., Goetz, M., Jain, S., Alsop, D., Camazine, S., andMeinwald, J. 1981. Antifeedant action ofZ-dilhydromatricaria acid from soldier beetles (Chauliognathus spp.)J. Chem. Ecol. 7:1149–1158.
Ficini, J., andDangelo, J. 1976. Synthese de la (±)isodihydronepetalactone et de deux de ses diastereoisomers.Tetrahedron Lett. 1976:687–690.
Grant, H.G., O'Regan, P.J., Park, R.J., andSutherland, M.D. 1980. Terpenoid chemistry XXIV. (1.R)-1-methoxymyodesert-3-ene, an iridoid constituent ofMyoporum deserti (Myoporaceae).Aust. J. Chem. 33:853–878.
Heller, S.R., andMilne, G.W.A. 1978. EPA/NIH Mass Spectral Data Base, Vol. 1. U.S. Government Printing Office, Washington, D.C.
Jenkins, M.F. 1957. The morphology and anatomy of the pygidial glands ofDianus coerulescens Gyllenhal (Coleoptera: Staphylinidae).Proc. Entomol. Soc. London 32A:159–168.
Kolbe, W., andProske, M.G. 1973.Iso-valeriansäure im Abwehrsekret vonZyras humeralis Grav. (Coleoptera, Staphylinidae).Entomol. Blätter 69:57–60.
Meinwald, J. 1954. The degradation of nepetalactone.J. Amer. Chem. Soc. 76:4571–4573.
Meinwald, J., Jones, T.H., Eisner, T., andHicks, K. 1977. New methylcyclopentanoid terpenes from the larval defensive secretion of a chrysomelid beetle (Plagiodera versicolora).Proc. Natl. Acad. Sci. U.S.A. 74:2189–2192.
Nakanishi, K., Goto, T., Ito, S., Natori, S., andNozoe, S. (eds.). 1974. Natural Products Chemistry, Vol. 1. Academic Press, New York, pp. 48–59.
Noirot, C., andQuennedey, A. 1974. Fine structure of insect epidermal glands.Annu. Rev. Entomol. 19:61–80.
Pasteels, J.M. 1968a. Les glandes tégumentaires des staphylins termitophiles. II. Les genresTermitellodes, Termella etNasutitella (Aleocharinae, Corotocini, Termitogastrina).Insectes Soc. 15:337–358.
Pasteels, J.M. 1968b. Le système glandulaire tégumentaire des Aleocharinae (Coleoptera, Staphylinidae) et son evolution chez des espèces termitophiles du genreTermitella.Arch. Biol. (Liege) 79:381–469.
Pasteels, J.M. 1969. Les glandes tégumentaires des staphylins termitophiles. III. Les aleocharinae des genresTermitophillus (Corotocini, Corotocina),Perinthodes, Catalina (Termitonannini, Perinthina),Termitusa (Termitohospitini, Termitusina).Insectes Soc. 16:1–26.
Remold, H. 1962. Über die biologische Bedeutung der Duftdrüsen bei Landwanzen (Geocorisae).Z. Vergl. Physiol. 45:636–694.
Robinson, R., Jaeger, R.H., andClark, K. 1962. 2,6-Dimethyloct-2-ene-1,8-dial.Chem. Abstr. Service 57:P2077e.
Sakan, T., Isoe, S., Hyeon, S.B., Katsumura, R., andMaeda, T. 1965. The exact nature of matatabilactone and the terpenes ofNepeta cataria.Tetrahedron Lett. 1965:4097–4102.
Schildknecht, H., Krauss, D., Connert, J., Essenbreis, H., andOrfanides, N. 1975. Das Spreitungsalkaloid Stenusin aus dem KurzflüglerStenus comma (Coleoptera: Staphylinidae).Angew. Chem. 87:421–422.
Stenhagen, E., Abrahamsson, S.A., andMcLafferty, F.W. 1974. Registry of Mass Spectral Data, Vol. 1. John Wiley & Sons, New York.
Tschinkel, W.R. 1975. A comparative study of the chemical defensive system of Tenebrionid beetles. Defensive behavior and ancillary features.Ann. Entomol. Soc. Am. 68:439–453.
Weatherston, J., andPercy, J.E. 1978. Venoms of coleoptera, pp. 511–554,in S. Bettini (ed.). Arthropod Venoms, Handbook of Experimental Pharmacology, Vol. 48. Springer-Verlag, Berlin.
Wheeler, J.W., Happ, G.M., Araujo, J., andPasteels, J.M. 1972. γ-Dodecalactone from rove beetles.Tetrahedron Lett. 1972:4635–4638.
Wolinsky, J., andEustace, E.J. 1972. Syntheses of the dihydronepetalactones.J. Org. Chem. 37:3376–3378.
Author information
Authors and Affiliations
Additional information
Paper No. 69 of the seriesDefense Mechanisms of Arthropods. Paper No. 68 is: Eisner, T., and Aneshansley, D.J. 1982.Science 215:83.
Rights and permissions
About this article
Cite this article
Jefson, M., Meinwald, J., Nowicki, S. et al. Chemical defense of a rove beetle (Creophilus maxillosus). J Chem Ecol 9, 159–180 (1983). https://doi.org/10.1007/BF00987779
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00987779