Abstract
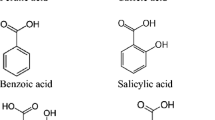
Allelopathy due to humus phenolics is a cause of natural regeneration deficiency in subalpine Norway spruce (Picea abies) forests. If inhibition of spruce germination and seedling growth due to allelochemicals is generally accepted, in contrast there is a lack of knowledge about phenolic effects on mycorrhizal fungi. Thus, this work tested effects of a humic solution and its naturally occurring phenolics on the growth and respiration of two mycorrhizal fungi: Hymenoscyphus ericae (symbiont of Vaccinium myrtillus, the main allelochemical-producing plant) and Hebeloma crustuliniforme (symbiont of P. abies, the target plant). Growth and respiration of H. crustuliniforme were inhibited by growth medium with the original humic solution (−6% and −30%), respectively, whereas the same humic solution did not affect growth but decreased respiration of H. ericae (−55%). When naturally occurring phenolics (same chemicals and concentrations in the original humic solution) were added to the growth medium, growth of H. crustuliniforme was not affected, whereas that of H. ericae significantly increased (+10%). We conclude that H. ericae is better adapted to the allelopathic constraints of this forest soil than H. crustuliniforme and that the dominance of V. myrtillus among understory species could be explained in this way.
Similar content being viewed by others
REFERENCES
AndrÉ, J., Gensac, P., Pellissier, F., and Trosset, L. 1987. Régénération des peuplements d'épicéa en altitude: Recherches préliminaires sur le rôle de l'allélopathie et de la mycorhization dans les premiers stades du développement. Rev. Ecol. Biol. Sol 24:301–310.
BENDING, G. D., and READ, D. J. 1997. Lignin and soluble phenolic degradation by ectomycorrhizal and ericoid mycorrhizal fungi. Mycol. Res. 101:1348–1354.
Boufalis, A., and Pellissier, F. 1994. Allelopathic effects of phenolic mixtures on the respiration of two spruce mycorrhizal fungi. J. Chem. Ecol. 20:2283–2289.
Boufalis, A., Pellissier, F., and Trosset, L. 1994. Responses of mycorrhizal fungi to allelopathy: Cenococcum geophilum and Laccaria laccata growth with phenolic acids. Acta Bot. Gall. 141:547–550.
Brossier, J. 1977. Evolution des idées en matière forestière et conséquences sur la gestion des forêts alpines. Rev. For. Fr. Waney 29:153–161.
Cairney, J. W. G., and Burke, R. M. 1994. Fungal enzymes degrading plant cell walls: Their possible significance in the ectomycorrhizal symbiosis. Mycol. Res. 98:1345–1356.
CotÉ, J. F., and Thibault, J. R. 1988. Allelopathic potential of raspberry foliar leachates on growth of ectomycorrhizal fungi associated with black spruce. Am. J. Bot. 75:966–970.
Duchaufour, P. 1953. Régénération de l'épicéa et pédologie. Rev. For. Fr. Nancy 4:257–268.
Fisher, R. F. 1987. Allelopathy: A potential cause of forest regeneration failure, pp. 176–184, in G. R. Waller (ed.). Allelochemicals: Role in Agriculture and Forestry. American Chemical Society Symposium Series, Washington, D.C.
Gallet, C. 1994. Allelopathic potential in bilberry-spruce forests: Influence of phenolic compounds on spruce seedlings. J. Chem. Ecol. 20:1009–1024.
Gallet, C., and Lebreton, P. 1995. Evolution of phenolic patterns from plants, litters and soils in a mountain bilberry-spruce forest. Soil Biol. Biochem. 27:157–165.
Gallet, C., and Pellissier, F. 1997. Phenolic compounds in natural solutions of a coniferous forest. J. Chem. Ecol. 23:2401–2412.
Giltrap, N. J. 1982. Production of polyphenol oxidases by ectomycorrhizal fungi with special reference to Lactarius spp. Trans. Br. Mycol. Soc. 78:75–81.
Handley, W. R. C. 1963. Mycorrhizal associations with Calluna heathland afforestation. Bull. For. Commun. 36:1–70.
Laiho, O. 1965. Further studies on the ectendotrophic mycorrhiza. Acta For. Fenn. 79:1–35.
Leake, J. R., and Read, D. J. 1989. The biology of the Ericaceae. XIII. Some characteristics of the extracellular proteinase activity of the ericoid endophyte Hymenoscyphus ericae. New Phytol. 112:69–76.
Mallik, A. U., and Zhu, H. 1995. Overcoming allelopathic growth inhibition by mycorrhizal inoculation, pp. 39–57, in Inderjit, K. M. Dakshini, and F. A. Einhellig (eds.). Allelopathy: Organisms, Processes and Prospects. American Chemical Society, Washington D.C.
Mayer, H. 1976. Die Verjüngung des Gebirdswaldes Schweiz. Z. Forstwes. 1:14–30.
Norkrans, B. 1949. Some mycorrhiza-forming Tricholoma species. Svensk. Bot. Tidsk. 43:485–490.
Pellissier, F. 1993a. Allelopathic inhibition of spruce germination. Acta Oecol. 14:211–218.
Pellissier, F. 1993b. Allelopathic effect of phenolic acids from humic solutions on two spruce mycorrhizal fungi: Cenococcum graniforme and Laccaria laccata. J. Chem. Ecol. 19:2105–2114.
Pellissier, F. 1994. Effects of phenolic compounds in humus on the natural regeneration of spruce. Phytochemistry 36:865–867.
Pellissier, F. 1996. Allelopathy from ecosystem to cell in spruce (Picea abies (L.) Karst.) forests, pp. 65–79, in S. S. Narwal and P. Tauro (eds.). Allelopathy: Field Observations and Methodology, Scientific Publishers, Jodhpur, India.
Pellissier, F., and Trosset, L. 1992. Les difficultés de régénération naturelle des pessières subalpines: Prédation des graines au sol et blocages dus à l'humus. Ann. Sci. For. 49:383–388.
Roques, A., and Trosset, L. 1986. Analyse de la distribution altitudinale des insectes ravageurs des cônes d'épicéa (Picea abies (L.) Karst.) dans les Alpes françaises du Nord, pp. 83–90, in A. Roques (ed.). Proceedings of the Second Conference on the Cone and Seed Insects. I.U.F.R.O., Besançon, France.
Rose, S. L., Perry, D. A., Pilz, D., and Schoeneberger, M. M. 1983. Allelopathic effects of litter on the growth and colonization of mycorrhizal fungi. J. Chem. Ecol. 9:1153–1162.
Schaeffer, A. 1911. Régénération de l'épicéa dans les forêts des Hautes Régions. Bull. Trimest. Soc. For. Franche-Comté et Belfort 11:292–300.
Smith, S. E., and Read, D. J. 1997. Mycorrhizal Symbiosis. Academic Press, London.
Souto, X. C., Chiapusio, G., and Pellissier, F. 1998. Soil microorganisms and phenolics: Their implication in spruce natural regeneration failure, pp. 301–308, in K. Sassa (ed.). Environmental Forest Science. Kluwer Academic Publishers, Dordrecht, The Netherlands.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Souto, C., Pellissier, F. & Chiapusio, G. Allelopathic Effects of Humus Phenolics on Growth and Respiration of Mycorrhizal Fungi. J Chem Ecol 26, 2015–2023 (2000). https://doi.org/10.1023/A:1005551912405
Issue Date:
DOI: https://doi.org/10.1023/A:1005551912405