Abstract
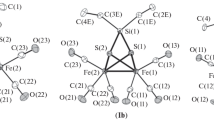
The reactions of K[Fe{Si(OMe)3}(CO)3(P∼Y)][P∼Y=Ph2PCH2C(O)Ph, Ph2PCH2C(O)[(η-C5H4)FeCp] (Cp=η5-C5H5), Ph2P(CH2)2CN] with CdCl2·2.5H2O, ZnX 2 (X=Cl, I) or InCl3 afforded Fe-Cd-Fe or Fe-M(μ-X)2 M-Fe (M=Cd, Zn, In;X=Cl, I) and Fe-InCl2 complexes. Some of them contain an unusual and labile μ-η2-SiO alkoxysilyl bridge which may be associated with a bridging mode for the ketophosphine ligand (first such example structurally established), thus providing original results in bimetallic chemistry on the intramolecular coordination of oxygendonor functions ofchemically different hemilabile ligands firmly attached to a neighboring metal center. The structures of the trinuclear complex
(3), of the chlorobenzene solvate of the tetranuclear complex
(4a·C6H5Cl) and of [mer-(OC)3{(EtO)3Si}
(4e) have been determined by X-ray diffraction. Crystals of 3 are orthorhombic, space groupPbcn, witha=19.010(4),b=11.766(5),c=26.998(7)Å, andZ=4. Crystals of4a·C6H5Cl are monoclinic, space groupC2/c witha=22.455(3),b=17.680(2),c=16.627(4)Å, β=90.80(4)°, andZ=4. Crystals of4e are monoclinic, space groupC2/c witha=25.392(5),b=18.554(6),c=16.28(1)Å, β=120.73(3)°, andZ=4. The structures were solved using direct methods and Fourier difference techniques and refined by blocked full-matrix least squares toR=0.035 (R w =0.049) for 2719 observed reflections, toR=0.042 (R w =0.056) for 3082 observed reflections, and toR=0.057 (R w =0.075) for 1850 observed reflections for3, 4a·C6H5Cl and4e, respectively. The Fe-Zn complexes
(9a),
(9b) and
(9c) were prepared and characterized by spectroscopic methods.
Similar content being viewed by others
References
A. Bader and E. Lindner (1991).Coord. Chem. Rev. 108, 27.
P. Braunstein, D. Matt, D. Nobel, S-E. Bouaoud, B. Carluer, D. Grandjean, and P. Lemoine (1986).J. Chem. Soc., Dalton Trans. 415;
P. Braunstein, D. Matt, and Y. Dusausoy (1983).Inorg. Chem. 22, 2043;
J. C. Jeffrey and T. B. Rauchfuss (1979) ibid.18, 2658.
P. Braunstein (1991).Mater. Chem. Phys. 29, 33.
P. Braunstein, M. Knorr, A. Tiripicchio, and M. Tiripicchio-Camellini (1989).Angew. Chem. 101, 1414; (1989).Angew. Chem. Int. Ed. Engl. 28, 1361;
P. Braunstein, M. Knorr, B. E. Villarroya, and J. Fischer (1990).New J. Chem. 14, 583;
P. Braunstein, M. Knorr, H. Piana, and U. Schubert (1991).Organometallics 10, 828;
P. Braunstein, M. Knorr, E. Villarroya, A. DeCian, and J. Fischer (1991). ibid.10, 3714;
P. Braunstein, M. Knorr, U. Schubert, M. Lanfranchi, and A. Tiripicchio (1991).J. Chem. Soc., Dalton Trans. 1507;
P. Braunstein, L. Douce, M. Knorr, M. Strampfer, M. Lanfranchi, and A. Tiripicchio (1992).ibid. 331;
P. Braunstein, E. Colomer, M. Knorr, A. Tiripicchio, and M. Tiripicchio-Camellini (1992).ibid. 903;
G. Reinhard, B. Hirle, U. Schubert, M. Knorr, P. Braunstein, A. DeCian, and J. Fischer (1993).Inorg. Chem. in press.
P. Braunstein, T. M. Gomes Carneiro, D. Matt, F. Balegroune, and D. Grandjean (1989).J. Organomet. Chem. 367, 117.
S. S. Al-Juaid, C. Eaborn, A. Habtemariam, P. B. Hitchcock, and J. D. Smith (1992).J. Organomet. Chem. 437, 41;
W. Wojnowski, B. Becker, L. Walz, K. Peters, E.-M. Peters, and H.-G. von Schnering (1992).Polyhedron 11, 607.
H. Adams, N. A. Bailey, D. E. Fenton, I. G. Ford, S. J. Kitchen, M. G. Williams, P. A. Tasker, A. J. Leong, and L. F. Lindoy (1991).J. Chem. Soc., Dalton Trans. 1665;
K. R. Adam, K. P. Dancey, B. A. Harrison, A. J. Leong, L. F. Lindoy, M. McPartlin, and P. A. Tasker (1983).J. Chem. Soc., Chem. Commun. 1351.
S. C. Goel, M. Y. Chiang, and W. E. Buhro (1990).J. Am. Chem. Soc. 112, 6724.
W. N. Setzer, Y. Tang, G. J. Grant, and D. G. VanDerveer (1992).Inorg. Chem. 31, 1116.
R. D. Ernst, T. J. Marks, and J. A. Ibers (1977).J. Am. Chem. Soc. 99, 2090;
ibid. (1977)99, 2098.
F. Teixidor, L. Escriche, I. Rodriguez, J. Casabo, J. Rius, E. Molins, B. Martinez, and C. Miravitlles (1989).J. Chem. Soc., Dalton Trans. 1381;
H. Leligny and J. C. Monier (1975).Acta Cryst. B31, 728 (and references cited).
C. Calvo and P. K. L. Au (1969).Can. J. Chem. 47, 3409;
W. Harrison and J. Trotter (1972).J.C.S. Dalton Trans. 956;
T. M. Greaney, C. L. Raston, A. H. White, and E. N. Maslen (1975).ibid. 876;
J. K. Shiba and R. Bau (1978).Inorg. Chem. 17, 3484;
A. Banerjee, C. J. Brown, P. C. Jain, and P. Gautam (1984).Acta Cryst. C40, 1161;
M. A. Romero, M. N. Moreno, J. Ruiz, M. P. Sanchez, and F. Nieto (1986).Inorg. Chem. 25, 1498;
R. Cini, G. Giorgi, A. Cinquantini, C. Rossi, and M. Sabat (1990).29, 5197;
S. Sogani, A. Singh, R. Bohra, R. C. Mehrotra, and M. Noltemeyer (1991).J. Chem. Soc., Chem. Commun. 738;
M. Nieuwenhuyzen, H. Wen, and C. J. Wilkins (1992).Z. Anorg. Allg. Chem. 615, 143.
R. G. Goel, W. P. Henry, and R. C. Srivastava (1981).Inorg. Chem. 20, 1727.
F. I. Aigbirhio, S. S. Al-Juaid, C. Eaborn, A. Habtemariam, P. B. Hitchcock, and J. D. Smith (1991).J. Organomet. Chem. 405, 149.
B. Neumüller (1989).Chem. Ber. 122, 2283;
H. J. Haupt, W. Wolff, and H. Preut (1976).Inorg. Chem. 15, 2920.
A. T. T. Hsieh and M. J. Mays (1972).J. Chem. Soc., Dalton Trans. 516;
L. M. Clarkson, N. C. Norman, and L. J. Farrugia (1991).Organometallics 10, 1286;
L. M. Clarkson, W. Clegg, D. C. R. Hockless, N. C. Norman, L. J. Farrugia, S. G. Bott, and J. L. Atwood (1991).J. Chem. Soc., Dalton Trans. 2241.
P. Braunstein, M. Knorr, M. Strampfer, A. DeCian, and J. Fischer (unpublished results).
M. Pfeffer, P. Braunstein, and J. Dehand (1974).Spectrochim. Acta 30A, 331.
D. H. Brown and D. T. Stewart (1970).J. Inorg. Nucl. Chem. 32, 3751.
B. N. Storhoff (1972).J. Organomet. Chem. 43, 197.
F. G. Mann and I. T. Miller (1952).J. Chem. Soc. 4453.
B. A. Frenz,in H. Schenk, R. Olthof-Hazekamp, H van Koningsveld, and G. C. Bassi (eds.),Computing in Crystallography (Delft University Press, Delft, 1978), pp. 64–71.
N. Walker and D. Stuart (1983).Acta Crystallogr. 39, 158.
International Tables for X-Ray Crystallography, Vol. 4 (Kynoch Press, Birmingham, 1974).
Author information
Authors and Affiliations
Additional information
Part 21 in the Series: Complexes with Functional Phosphines. Part 20: P. Braunstein, S. Coco Cea, A. DeCian, and J. Fischer (1992).Inorg. Chem. 31, 4203.
Rights and permissions
About this article
Cite this article
Balegroune, F., Braunstein, P., Douce, L. et al. Synthesis and crystal structures of heterobimetallic Fe-Cd, Fe-Zn, and Fe-In complexes containing hemilabile phosphorus/oxygen and silicon/oxygen bridging ligands. J Clust Sci 3, 275–296 (1992). https://doi.org/10.1007/BF01028547
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01028547