Abstract
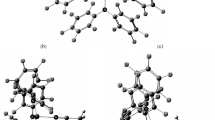
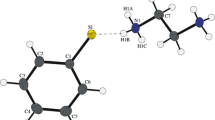
The crystal structure of the donor — acceptor adduct (CH3)2HN−SO2 is reported. Crystals of the compound are clear prisms, space group Pna21; at −96°C,a=9.894(1),b=6.051(2),c=16.478(3) Å,D=1.469 g/cm3,V=786.6(5) Å,Z=8. The unit cell contains two crystallographically independent adducts, with N−S bond lengths of 2.015(3) Å and 1.991(3) Å. The average value, 2.00(1) Å is 0.33(4) Å shorter than the 2.34(3) Å value previously determined from microwave spectroscopy of the gas phase species, revealing a large medium effect on the length of the donor — acceptor bond. The structural results are compared with published data for the trimethylamine — SO2 adduct, and discussed in terms of existing experimental and theoretical results for other donor — acceptor systems.
Similar content being viewed by others
References
Dvorak, M.A.; Ford, R.S.; Suenram, R.D.; Lovas, F.J.; Leopold, K.R.J. Am. Chem. Soc. 1992,114, 108.
Reeve, S.W.; Burns, W.A.; Lovas, F.J.; Suenram, R.D.; Leopold, K.R.J. Phys. Chem. 1993,97, 10630.
Burns, W.A.; Leopold, K.R.J. Am. Chem. Soc. 1993,115, 11622.
Canagaratna, M.; Phillips, J.A.; Goodfriend, H.; Leopold, K.R.J. Am. Chem. Soc. 1996,118, 5290.
Leopold, K.R.; Fraser, G.T.; Novick, S.E.; Klemperer, W.Chem. Rev. 1994,94, 1807.
Leopold, K.R. InAdvances in Molecular Structure Research, Vol. II: Hargittai, M.; Hargittai, I.; Eds.; JAI Press, 1996; p 103.
Oh, J.J.; LaBarge, M.S.; J. Matos, J.; Kampf J.W.; Hillig, II, K.W.; Kuczkowski, R.L.J. Am. Chem. Soc. 1991,113, 4732.
van der Helm, D.; Childs, J.D.; Christian, S.D.Chem. Commun.1969, 887.
see, for example, Grundnes, J.; Christian, S.D.; Cheam, V.; Farnham, S.B.J. Am. Chem. Soc. 1971,93, 20.
Scott, W.D.; Lamb, D.J. Am. Chem. Soc. 1970,92, 3943.
Scott, W.D.; Lamb, D.; Duffy, D.J. Atmos. Sci. 1969,26, 727.
Grundnes, J.; Christian, S.D.J. Am. Chem. Soc. 1968,90, 2239.
Moede, J.A.; Curran, C.J. Am. Chem. Soc. 1949,71, 852.
Sass, C.S.; Ault, B.S.J. Phys. Chem. 1984,88, 432.
Nord, L.J. Mol. Struct. 1982,96, 27.
Hisatsune, I.C.;Heicklen, J.Can. J. Chem. 1975,53, 2646.
Byrd, W.E.;Inorg. Chem. 1962,1, 762.
Pradeep, T.; Sreekanth, C.S.; Hegde, M.S.; Rao, C.N.R.;J. Am. Chem. Soc. 1989,111, 5058.
Wu, K.T.; Yencha, A.J.Can. J. Chem. 1981,59, 8.
Wong, M.W.; Wiberg, K.B.J. Am. Chem. Soc. 1992,114, 7527.
Sakaki, S.; Sata, H.; Imai, Y.; Morokuma, K.; Katsutoshi, O.Inorg. Chem. 1985,24, 4538.
Douglas, J.E.; Kollman, P.A.J. Am. Chem. Soc. 1978,100, 5226.
Lucchese, R.R.; Haber, K.; Schaefer, III, H.F.J. Am. Chem. Soc. 1976,98, 7617.
Oh, J.J.; Hillig, II, K.W.; Kuczkowski, R.L.J. Phys. Chem. 1991,95, 7211.
Gillmore, C.J.J. Appl. Cryst. 1984,17, 42.
Molecular Structure Corporation (1985)TEXAN.TEXRAY Structure Analysis Package: MSC, 3200A Research Forest Drive, The Woodlands, TX 77381.
Beurskens, P.T. DIRDIF:Direct methods for difference structures. Technical Report 1984/1, Crystallography Laboratory: Toernooiveld, 6525 Ed Nijmegen, Netherlands.
Hargittai, I.; Hargittai, M. InMolecular Structure and Energetics VCH Publishers, Inc.1987; p 1.
Hargittai, M.; Hargittai, I.Phys. Chem. Miner. 1987,14, 413, and references therein.
Pauling, L.C.The Nature of the Chemical Bond: Cornell University Press: Ithaca, NY 1960.
It is worth noting that the 1.74 Å prediction agrees well with the 1.77 Å value observed in crystalline sulfamic acid (H3N−SO3) [21], indicating that the standard covalent radii are applicable to these datively bonded complexes.
Kanda, F.A.; King, A.J.J. Am. Chem. Soc. 1951,73, 2315.
Sass, R.L.Acta Crystallogr. 1960,13, 320.
Bats, J.W.; Coppens, P.; Koetzle, T.F.Acta Crystallogr. 1977,B33, 37.
Morris, A.J.; Kennard, C.H.L.; Hall, J.R.Inorg. Chim. Acta 1982,62, 247.
Jiao, H.; Schleyer, P. von R.J. Am. Chem. Soc. 1994,116, 7429.
Wong, M.W.; Wiberg, K.B.; Frisch, M.J.J. Am. Chem. Soc. 1992,114, 523.
Bühl, M.; Steinke, T.; Schleyer, P. von R.; Boese, R.Angew. Chem. Int. Ed. Engl. 1991,30, 1160.
We note that the nearly anti-parallel arangement of adjacent DMA-SO2 units, which is evident in Figs. 1 and 2, will be particularly conducive to cooperative dipolar interactions.
In this approach, the environment about a “solute” molecule is modeled as a dielectric continuum, whose response to its polar “guest” is to itself polarize, thus introducing a perturbation on the molecular and electronic structure of the solute.Ab initio calculations within the polarized medium are iterated until self consistency is obtained, and comparison with the gas phaseab initio structure gives the gas — “solid” structure difference.
Childs, J.D.; van der Helm, D.; Christian, S.D.Inorg. Chem. 1975,14, 1386.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Phillips, J.A., Britton, D. & Leopold, K.R. Gas-solid structure differences in the donor-acceptor complex (CH3)2HN−SO2 . J Chem Crystallogr 26, 533–538 (1996). https://doi.org/10.1007/BF01668411
Issue Date:
DOI: https://doi.org/10.1007/BF01668411