Abstract
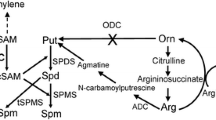
S-adenosylmethionine decarboxylase (SAMDC; EC 4.1.4.50) is one of the key enzymes in polyamine biosynthesis, and the product of its catalytic reaction, decarboxylated S-adenosylmethionine (dcSAM), serves as an aminopropyl donor in the biosynthesis of spermidine and spermine. In order to provide information on the structure and regulation of SAMDC, we have isolated and sequenced two different SAMDC cDNA clones from carnation petals. The nucleotide sequences of CSDC9 and CSDC16 show 78.3% identity, and the deduced amino acid sequences show 81.7% identity and 86.5% similarity [12]. There are several regions with highly conserved sequences among SAMDC cDNAs of potato, spinach, periwinkle, man and yeast. These conserved regions include a cleavage site for the processing of SAMDC proenzyme and a putative PEST sequence that may be relevant to the rapid degradation of SAMDC protein. Carnation SAMDC cDNAs have long transcript leaders of 472 bp and 502 bp for CSDC9 and CSDC16, respectively. Both sequences contain short upstream open reading frames (uORFs) in their 5′ -untranslated regions. The CSDC9 uORF is 54 amino acids from 152 to 317 while the corresponding sequence in CSDC16 is 52 amino acids located from 156 to 314 in each 5′-untranslated region. The nucleotide sequences of uORFs in CSDC9 and CSDC16 were 89.9% identical. In vitro transcription/translation experiments showed: (1) each proenzyme of both cDNAs of SAMDC was converted to two polypeptides consisting of a large subunit (calculated as 31544 Da and 32537 Da, respectively) and a small subunit (calculated as 9704 and 9041 Da, respectively) after 20 min of translation; (2) the processing occurs rapidly during the translation of protein. But once the translation process is stopped accumulation of the subunits slows and never reaches completion even after 300 min. The processing of carnation SAMDC enzyme is not stimulated by putrescine in in vitro transcription/translation reaction.
Similar content being viewed by others
References
Baxter C, Coscia CJ: In vitrosynthesis of spermidine in the higher plant, Vinca rosea. Biochem Biophys Res Commun 54: 147-154 (1973).
Bolle C, Herrmann RG, Oelmüller R: A spinach cDNA with homology to S-adenosylmethionine decarboxylase. Plant Physiol 107: 1461-1462 (1995).
Chirgwin JM, Pryzbyla AE, Macdonald RJ, Rutter WJ: Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 18: 5249-5299 (1979).
Cohn MS, Tabor CW, Tabor H: Identification of a pyruvoyl residue in S-adenosylmethionine decarboxylase from Saccharomyces cerevisiae. J Biol Chem 252: 8212-8216 (1977).
Dellaporta SL, Wood J, Hicks JB: A plant DNA miniprep: Version II. Plant Mol Biol Rep 1: 19-21 (1983).
Evans PT, Malmberg RL: Do polyamines have roles in plant development? Annu Rev Plant Mol Biol 40: 235-269 (1989).
Hammill JD, Robins, RJ, Parr AJ, Evans DM, Furze JM, Rhodes, MJC: Over-expressing a yeast ornithine decarboxylase gene in transgenic roots of Nicotiana rusticacan lead to enhanced nicotine accumulation. Plant Mol Biol 15: 27-38 (1992).
Hill JR, Morris DR: Cell-specific translation of Sadenosylmethionine decarboxylase mRNA. J Biol Chem 267: 21-886-21-893 (1992).
Hunt T: False starts in translational control of gene expression. Nature 316: 581-582 (1985).
Kahana C, Nathans D: Nucleotide sequence of murine ornithine decarboxylase mRNA. Proc Natl Acad Sci USA 82: 1673- 1677 (1985).
Kashiwagi K, Taneja SK, Liu TY, Tabor CW, Tabor H: Spermidine biosynthesis in Saccharomyces cerevisiae: biosynthesis and processing of a proenzyme form of a Sadenosylmethionine decarboxylase. J Biol Chem 265: 22-321- 22-328 (1990).
Lee MM, Lee SH, Park KY: Nucleotide sequence of cDNA (accession No. U38526, U38527) encoding Sadenosylmethionine decarboxylase from carnation flower (PGR 95-139). Plant Physiol 110: 714 (1996).
Lee MM: cDNA cloning of S-adenosylmethionine decarboxylase from carnation (Dianthus caryophyllusL.) petal and studies on the expression. Ph. D. thesis, Yonsei University, Seoul (1996).
Mad Arif SA, Taylor MA, George LA, Butler AR, Burch LR, Davies HV, Stark MJR, Kumar A: Characterisation of the Sadenosylmethionine decarboxylase (SAMDC) gene of potato. Plant Mol Biol 26: 327-338 (1994).
Marié SC, Crozat A, Jänne OA: Structure and organization of the human S-adenosylmethionine decarboxylase gene. J Biol Chem 267: 18-915-18-923 (1992).
Marton LJ, Morris DR: Molecular and cellular functions of the polyamines. In: McCann PP, Pegg AE, Sjoerdsma A (eds) Inhibition of Polyamine Biosynthesis: Biological Significance and Basis for New Therapies, pp. 79-105. Academic Press, New York (1987).
Pajunen A, Crozat A, Jänne OA, Ihalainen R, Laitinen PH, Stanley B, Madhubala R, Pegg AE: Structure and regulation of mammalian S-adenosylmethionine decarboxylase. J Biol Chem 263: 17-040-17-049 (1988).
Park KY, Lee SH: Effects of ethylene and auxin on polyamine levels in suspension-cultured tobacco cells. Physiol Plant 90: 382-390 (1994).
Park KY, Lee SH: Role of S-adenosylmethionine as an intermediate in relation between polyamine and ethylene biosynthesis in suspension-cultured tobacco cells. Korean J Bot 33: 87-96 (1990).
Pegg AE, McCann PP: Polyamine metabolism and function. Am J Physiol 12: C210-C221 (1982).
Perez-Amador MA, Carbonell J, Granell A: Expression of arginine decarboxylase is induced during early fruit development and in young tissue of Pisum sativum(L.). Plant Mol Biol 28: 997-1009 (1995).
Rogers S, Wells R, Rechsteiner M: Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science 234: 364-368 (1986).
Schröder G, Schröder J: cDNAs for S-adenosylmethionine decarboxylase from Catharanthus reseus, heterologous expression, identification of proenzyme-processing site, evidence for the presence of both subunits in the active enzyme, and a conserved region in the 5′ mRNA leader. Eur J Biochem 228: 74-78 (1995).
Shantz LM, Viswanath R, Pegg AE: Role of the 5′-untranslated region of mRNA in the synthesis of S-adenosylmethionine decarboxylase and its regulation by spermine. Biochem J 302: 765-772 (1994).
Stanley BA, Pegg AE, Holm I: Site of pyruvate formation and processing of mammalian S-adenosylmethionine decarboxylase proenzyme. J Biol Chem 264: 21-073-21-079 (1989).
Tabor CW, Tabor H: Polyamines. Annu Rev Biochem 53: 749- 790 (1984).
Taylor MA, Mad Arif SA, Kumar A, Davies HV, Scobie LA, Pearce SR, Flavell AJ: Expression and sequence analysis of cDNAs induced during the early stages of tuberisation in different organs of potato plant (S. tuberosumL.). Plant Mol Biol 20: 641-651 (1992).
Werner M, Feller A, Messinguy F, Piarard A: The leader peptide of yeast gene CPA1 is essential for the translational repression of its expression. Cell 49: 805-813 (1987).
White MW, Morris DR: S-adenosylmethionine decarboxylase genes and expression. In: Bachrach U, Heimer YM (eds) The Physiology of Polyamines, pp. 331-343. CRC Press, Boca Raton, FL (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lee, M.M., Lee, S.H. & Park, K.Y. Characterization and expression of two members of the S-adenosylmethionine decarboxylase gene family in carnation flower. Plant Mol Biol 34, 371–382 (1997). https://doi.org/10.1023/A:1005811229988
Issue Date:
DOI: https://doi.org/10.1023/A:1005811229988