Abstract
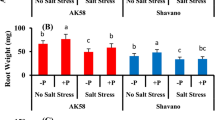
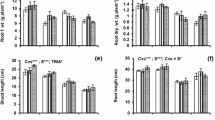
Wheat plants, 22d. old, were exposed to wide range of soil water osmotic potential (Ψs = 0 to −1.2 MPa) induced by NaCl and CaCl2 treatments in combination with roots maintained under aerobic (drained at field capacity) or nonaerobic (flooded) conditions in the soil, and sprayed with 10 mg L−1 kinetin solution. In drained plants, not receiving kinetin, increased soil salinity resulted in appreciable inhibition of shoot growth and reduction in chlorophyll (Ch1.), soluble sugars (SS) contents and grain yield. Shoot growth, Ch1. content, soluble sugars and grain yield were significantly lower for flooded plants than unflooded analogues over the entire Ψs range. Both salinity and waterlogging synergize to increase Na+, Ca+ and Cl− accumulation in shoot tissues and to decrease the stability of leaf membranes to either dehydration (40% polyethylene glycol 6000) or heat (51 °C) stress. The ratio of K+/Na+ transported to shoots under aerobic and anaerobic conditions decreased progressively on salinization. The association between the internal mineral element concentrations was largely affected by kinetin treatment. Kinetin application ameliorated the deleterious effects of salinity and oxygen deficiency. It reduced Na+, Ca2+ and Cl− accumulation and improved K+ uptake under salinity and waterlogging stresses. Increased K+/Na+ ratio helped the plants to avoid Na+ toxicity and enhanced shoot growth and grain yield. Kinetin also reduced membrane injury by dehydration and heat stresses and improved the water status of plants under both aerobic and anaerobic conditions. The effects of single factors (Soil salinity ‘Ψs’, soil waterlogging ‘WL’ and Kinetin ‘Kin’) and their interactions (Ψs × WL, Ψs × Kin, WL × Kin and Ψs × WL × Kin) were shown by analysis of variance to be statistically significant for most parameters tested. Calculation of the coefficient of determination (η+) led to three important findings. (1) Salinity (Ψs) was dominant in affecting leaf relative water content (RWC), shoot dry mass, grain yield, stability of leaf membranes to dehydration stress and the contents of Na+, Ca2+, Mg2+ and Cl−. (2) Kinetin (Kin) had a dominant effect on the stability of leaf membranes to heat stress as well as on chlorophyll and soluble sugars contents. (3) The share of waterlogging (WL) was dominant for K+ content. It can be concluded that kinetin application helped wheat plants to grow successfully in the areas subjected to combined effects of salinity and oxygen deficiency, such as in salt marshes.
Similar content being viewed by others
References
Armstrong W, Brandle R and Jackson MB (1994) Mechanisms of flood tolerance in plants. Acta Bot Neer 43: 307–358
Benlloch M, Ojeda MA, Ramos J and Rodriquez, A (1994) Salt sensitivity and low discrimination between potassium and sodium in bean plants. Plant and Soil 166: 117–123
Bishoni NR and Krishnamoorthy HN (1992) Effect of waterlogging and gibberellic acid on leaf gas exchange in peanut (Arachis hypogaea L.). Plant Physiol 139: 503–505
Blum A and Ebercon A (1981) Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci 21: 43–47
Bohra JS and Dörffling K (1993) Potassium nutrition of rice (Oryza sativa L.) varieties under NaCl salinity. Plant and Soil 152: 299–303
Botella MA, Martinez V, Pardines J and Cerda A (1997) Salinity induced potassium deficiency in maize plants. J Plant Physiol 150: 200–205
Brignoli E and Lauteri M (1991) Effect of salinity on stomatal conductance, photosynthetic capacity and carbon isotope discrimination of salt-tolerant (Gossypium hirsutum L.) and salt senstive (Phaseolus vulgaris L) C3 non. halophytes. Plant Physiol 95: 628–635
Burrows WJ and Carr DJ (1969) Effects of flooding the root system of sunflower plants on the cytokinin content of the xylem sap. Plant Physiol 22: 1105–1112
Buysse J and Merckx R (1993) An improved colorimetric method to quantify sugar content of plant tissue. J Exp Bot 44: 1627–1629
Chang PFL, Damsz B, Kononowicz AK, Reuveni M, Chen Z, Yixu Hedges K, Tseng CC, Singh NK, Binzel ML, Narasimhan ML, Hasegawa PM and Bressan RA (1996) Alteration in cell membrane structure and expression of a membrane-associated protein after adaptation to osmotic stress. Physiol Plant 98: 505–516
Cheeseman J and Hansan JB (1979) Energy-linked potassium influx as related to cell potential in corn roots. Plant Physiol 64: 842–845
Chow WS, Ball MC and Anderson JM (1990) Growth and photosynthetic response of spinach to salinity. Implication of K+ nutrition for salt tolerance. Aust J Plant Physiol 17: 553–578
Cooper A (1982) The effect of salinity and waterlogging on the growth and cation uptake of salt marsh plant. New Phytol 90: 263–275
Davenport RJ, Reid RJ and Smith FA (1997) Sodium-calcium interactions in two wheat differing in salinity tolerance. Physiol Plant 99: 323–327
Davies MS and Hillman C (1988) Effect of soil flooding on growth and grain yield of population of tetraploid and hexaploid species of wheat. Ann Bot 62: 597–604
Drew MC (1990) Sensing soil oxygen. Plant Cell Envir 13: 681–693
Drew MC (1991) Oxygen deficiency in the root environment and plant mineral nutrition. In: Jackson MB, Davies DD and Lambers H (eds) Plant Life under Oxygen Deprivation, pp 303–316. The Hague, The Netherlands: Academic Publishing
Everard JD and Drew MC (1989) Water relations of sunflower (Helianthus annuus) shoots during exposure of the root system to oxygen deficiency. J Exp Bot 40: 1255–1264
Gadallah MAA (1994) The combined effects of acidification stress and kinetin on chlorophyll content, dry matter accumulation and transpiration coefficient in Sorghum bicolor plants. Biol Plant 36: 149–153
Gadallah MAA (1995a) Effect of waterlogging and kinetin on the stability of leaf membranes, leaf osmotic potential, soluble carbon and nitrogen compounds and chlorophyll content of Ricinus plants. Phyton 35: 199–208
Gadallah MAA (1995b) Effect of cadmium and kinetin on chlorophyll content, saccharides and dry matter accumulation in sunflower plants. Biol Plant 37: 233–240
Gadallah MAA (1996) Abscisic acid, temperature and salinity interactions on growth and some mineral elements in Carthamus plants. J Plant Growth Regul 20: 225–236
Gadallah MAA and Ramadan T (1997) Effects of zinc and salinity on growth and anatomical structure of Carthamus tinctorius L. Biol Plant 39: 411–418
Gorham J, Bristol A, Young EM, Wyn Jones RG and Kashour G (1990) Salt tolerance in the triticeae: K+/Na+ discrimination in barley. J Exp Bot 41: 1095–1101
Guglielminetti L, Wu Y, Boschi E, Yamaguchi J, Favatt A, Vergara M, Perata P and Alpi A (1997) Effects of anoxia on sucrose degrading enzymes in cereal seeds. J Plant Physiol 150: 251–258
Hanhijarvi AM and Fagerstedt KV (1995) Comparison of carbohydrate utilization and energy charge in the yellow flag iris (Iris pseudacorus) and garden iris (Iris germanica) under anoxia. Physiol Plant 93: 493–497
He T and Cramer GR (1993) Salt tolerance of rapid-cycling Brassica species in relation to potassium sodium ratio and selectivity at the whole plant and callus levels. J Plant Nutr 16: 1263–1277
Huang B, Johnson JW, Nesmith S and Bridges CD (1994) Growth, physiological and anatomical responses of two wheat genotypes to waterlogging and nutrient supply. J Exp Bot 45: 193–202
Jeschke WD (1984) K+-Na+ exchange at cellular membranes, interacellular compartmentation of cations, and salt tolerance. In: Staples RC and Toenniessen J (eds) In Salinity Tolerance in Plants, pp. 37–66. New York: Wiley
Johnson CM and Ulrich A (1959) Analytical methods for use in plant analysis. US Department of Agriculture. California University of Agricultural Information Bulletin
Läuchli A and Epstein E (1990) Plant responses to saline and sodic conditions: In: Tanyi KK (ed) Agricultural Salinity Assessment and Management, pp 133–137. New York: American Society of Civil Engineers
Mohanty B, Wilson PM and Res T (1993) Effects of anoxia on growth and carbohydrate metabolism in suspension cultures of soybean and rice. Phytochemistry 34: 75–82
Naidoo G, Mckee KL and Mendelssohn IA (1992) Anatomical and metabolic responses to waterlogging and salinity in Spartina alterniflora L. and Spartina potens A. Amer J Bot 79: 765–770
Niu X, Bressan RA, Hasegawa PM and Pardo JM (1995) Ion homeostasis in NaCl stress environments. Plant Physiol 109: 735–742
Ostle B (1963) Statistics in Research. Amer, Iowa, USA: The Iowa State University Press
Perata P, Pozueta-Romero J, Akazawa T and Yamaguchi J (1992) Effect of anoxia on starch breakdown in rice and wheat seeds. Planta 188: 611–618
Perata P, Guglielminetti L and Alpi A (1996) Anaerobic carbohydrate metabolism in wheat and barley, two anoxia-intolerant cereal seeds. J Exp Bot 47: 999–1006
Premachandra GS, Saneoka H, Fujita K and Ogata S (1992) Osmotic adjustment and stomatal response to water deficits in maize. J Exp Bot 43: 1451–1456
Reece CP and Riha SJ (1991) Role of root systems of eastern larch and white spruce in response to flooding. Plant Cell Envir 14: 229–234
Salama FM and Awadalla AA (1987) The effects of different kinetin application methods on some chlorophyll parameters of two crop plants grown under salinity stress. Phyton 21: 181–193
Serrato Valenti G, Melone L, Orsi O and Riverost S (1992) Anatomical changes in Prosopis cineraria (L.) Druce seedlings growing at different levels of NaCl salinity. Ann Bot 70: 399–404
Singh BP, Tucker KL, Sutton JD and Bhardwai HL (1991) Flooding reduces gas exchange and growth in snap bean. Hort Science 26: 372–373
Smith RCG, Mason WK, Meyer WS and Barrs HD (1983) Irrigation in Australia: development and future prospects. Advances in Irrigation 2: 99–153
Tal M and Shannon MC (1983) Salt tolerance in the wild relatives of the cultivated tomato: Response of Lycopersicum esculentum, L. Checsmoni, L. peruvianum, Solanum pennellii and F1 hybrids to high salinity. Aust J Plant Physiol 10: 109–117
Tood GW and Basler E (1965) Fate of various protoplasmic constituents in droughted wheat plants. Phyton 22: 79–85
Van der Moezel PG, Watson LE and Bell DT (1989) Gas exchange responses of two Eucalyptus species to salinity and waterlogging. Tree Physiol 5: 251–257
Visser EJW, Heijink CJ, Van Hout KJGM, Voesenek LACJ, Barendse GWM and Blom CWPM (1995) Regulatory role of auxin in adventitious root formation in two species of Rumex, differing in their sensitivity to waterlogging. Physiol Plant 93: 116–122
Wainwright SJ (1984) Adaptations of plants to flooding with salt water. In: Kozlowsk TT (ed) Flooding and Plant Growth, pp 295–343. New York: Academic Press
Wample RL and Thornton RK (1984) Differences in the response of sunflower (Helianthus annuus) subjected to flooding and drought stress. Physiol Plant 61: 611–616
Weatherley PE and Barrs C (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15: 413–428
Williams S and Hester P (1983) Kinetin increases water permeability of phosphatidylcholine lipid biolayers. Plant Physiol 71: 524–530
Yamada Y, Hara Y, Katagh H and Senda M (1980) Protoplast fusion, the effect of low temperature on the membrane fluidity of cultured cells. Plant Physiol 65: 1098–1102
Yeo AR, Caporn SJM and Flowers TJ (1985) The effect of salinity upon photosynthesis in rice (Oryza sativa L): gas exchange by individual leaves in relation to their salt content. J Exp Bot 36: 1240–1243
Yeo AR, Yeo ME and Flowers TJ (1987) The contribution of an apoplastic pathway to sodium uptake by rice roots in saline conditions. J Exp Bot 38: 1141–1163
Zhang J and Zhang X (1994) Can early wilting of old leaves account for much of the ABA accumulation in flooded pea plants? J Exp Bot 45: 1335–1342
Zhang Q and Greenway H (1994) Anoxia tolerance and anaerobic catabolism of aged beetroot storage tissues. J Exp Bot 45: 567–575
Zhang Q, Läuchli A and Greenway H (1992) Effects of anoxia on solute loss from beetroot strorage tissues. J Exp Bot 43: 897–905
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gadallah, M. Effects of kinetin on growth, grain yield and some mineral elements in wheat plants growing under excess salinity and oxygen deficiency. Plant Growth Regulation 27, 63–74 (1999). https://doi.org/10.1023/A:1006181204765
Issue Date:
DOI: https://doi.org/10.1023/A:1006181204765