Abstract
This study was conducted to evaluate the effects of different concentrations of dietary lipids on body composition and liver function in juvenile red drum, Sciaenops ocellatus. Diets were formulated to contain 40% crude protein from solvent-extracted menhaden fish meal and 0, 7, 14 or 21% lipid from menhaden fish oil. The basal diet, without supplemental fish oil, contained lipid at 0.4% of dry weight. The diets were fed to groups of 25 juvenile red drum initially averaging 7.3 ± 0.18 g fish−1 in a recirculating culture system for 8 weeks and weight gain was recorded. After an additional 8 weeks, 16 fish from each treatment were sacrificed and the following measurements were recorded: hepatosomatic index (HSI), intraperitoneal fat (IPF) ratio, and liver α-tocopherol, malondialdehyde (MDA) formation, and cytochrome P-4501A activity (measured as 7-ethoxyresorufin O-deethylase (EROD) activity). The activity of alanine and aspartate aminotransferases and concentrations of α-tocopherol also were measured in plasma.
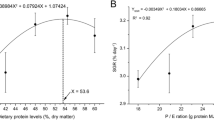
Weight gain was significantly (p<0.05) affected by dietary lipid concentration, with values ranging from 361% of initial weight for fish fed the basal diet to 527% of initial weight for fish fed the diet containing 7% lipid. The HSI and IPF ratio values also were significantly affected by lipid with the lowest values recorded for fish fed the basal diet and the highest values observed in fish fed the diet containing 21% lipid. Increasing dietary lipid significantly increased oxidative stress as reflected in reduced α-tocopherol in liver and plasma and increased MDA formation in the liver, although no overt pathological signs were observed. These findings suggest that lipid concentrations between 7 and 14%, when the diet contains 60 IU vitamin E kg−1, are likely to limit oxidative stress and result in normal physiological responses of red drum.
Similar content being viewed by others
References
Ammouche, A., Dinh, L., Youyou, A., Clement, M. and Bourre, J.M. 1993. Rate of alteration of hepatic mixed-function oxidase system in rats fed different dietary fats. Biochem. Cell Biol. 71: 530–537.
Ankley, G.T., Blazer, V.S., Plakas, S.M. and Reinert, R.E. 1989. Dietary lipid as a factor modulating xenobiotic metabolism in channel catfish (Ictalurus punctatus). Can. J. Fish. Aquat. Sci. 46: 1141–1145.
Bai, S.C. and Gatlin, D.M., III. 1992. Dietary vitamin E concentration and duration of feeding affect tissue α-tocopherol concentrations of channel catfish (Ictalurus punctatus). Aquaculture 113: 129–135.
Bus, J.S. and Gibson, J.E. 1979. Lipid peroxidation and its role in toxicology. In: Reviews in Toxicology. Vol. 1, pp. 125–148. Edited by E. Hodgen, J.R. Bend and R.M. Philpot. Elsevier North Holland, Inc., New York.
Castell, J.D., Lee, D.J. and Sinnhuber, R.O. 1972. Essential fatty acids in the diet of rainbow trout (Salmo gairdneri): lipid metabolism and fatty acid composition. J. Nutr. 102: 93–100.
Cowey, C.B., Adron, J.W., Walton, M.J., Murray, J., Youngson, A. and Knox, D. 1981. Tissue distribution, uptake and requirement for α-tocopherol of rainbow trout (Salmo gairdneri) fed diets with a minimum content of unsaturated fatty acids. J. Nutr. 111: 1556–1567.
Cowey, C.B., Adron, J.W. and Youngson, A. 1983. The vitamin E requirement of rainbow trout (Salmo gairdneri), given diets containing polyunsaturated fatty acids derived from fish oil. Aquaculture 30: 85–93.
Craig, S.R. 1994. Dietary manipulation of lipid deposition and cold tolerance in juvenile red drum, Sciaenops ocellatus. Doctoral dissertation. Texas A&M University, College Station, Texas.
Craig, S.R. and Gatlin, D.M., III. 1995. Coconut oil and beef tallow, but not tricaprylin, can replace menhaden oil in the diet of red drum (Sciaenops ocellatus) without adversely affecting growth or fatty acid composition. J. Nutr. 125: 3041–3048.
Craig, S.R., Neill, W.H. and Gatlin, D.M., III. 1995. Effects of dietary lipid and environmental salinity on growth, body composition, and cold tolerance of juvenile red drum (Sciaenops ocellatus). Fish Physiol. Biochem. 14: 49–61.
Dharwadkar, S.M., and Wade, A.E. 1987. BP metabolism and the in vivo binding to hepatic DNA of rats fed diets containing menhaden fish oil. Nutr. Cancer 10: 163–170.
Draper, H.H., Squires, E.J., Mahmood, H., Wu, J., Agarwal, S. and Hadley, M. 1993. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Rad. Biol. Med. 15: 353–363.
Erickson, M.C. 1992. Lipid and tocopherol composition of farmraised striped and hybrid striped bass. Comp. Biochem. Physiol. 101A: 171–176.
Flood, L.P., Carvan, M.J., III., Jaeger, L., Busbee, D.L., Gatlin, D.M., III., and Neill, W.H. 1996. Reduction in hepatic microsomal P-450 and related catalytic activity in farm-raised red drum. J. Aquat. Anim. Health 8: 13–21.
Folch, J., Lees, M. and Sloane-Stanley, G.H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509.
Gatlin, D.M., III. 1995. Review of red drum nutrition. In: Nutrition and Utilization Technology in Aquaculture. pp. 41–49. Edited by C.E. Lim and D.J. Sessa. AOCS Press, Champaign.
Hanley, F. 1991. Effects of feeding supplementary diets containing varying levels of lipid on growth, food conversion, and body composition of Nile tilapia, Oreochromis niloticus (L). Aquaculture 93: 323–334.
Ketola, H.G. 1976. Choline metabolism and nutritional requirements of lake trout (Salvelinus namaycush). J. Animal Sci. 43: 474–477.
Lochmann, R.T. and Gatlin, D.M., III. 1993a. Evaluation of different types and levels of triglycerides, singly and in combination with different levels of n-3 highly unsaturated fatty acid ethyl esters in diets of juvenile red drum (Sciaenops ocellatus). Aquaculture 114: 113–130.
Lochmann, R.T. and Gatlin, D.M., III. 1993b. Essential fatty acid requirement of juvenile red drum, Sciaenops ocellatus. Fish Physiol. Biochem. 12: 221–235.
Mihaich, E.M. and Di Guilio, R.T. 1986. Antioxidant enzyme activities and malondialdehyde, glutathione and methemoglobin concentrations in channel catfish exposed to DEF and N-butyl mercaptan. Comp. Biochem. Physiol. 85C: 427–432.
Murata, H. and Yamauchi, K. 1989. Relationship between the 2-thiobarbituric acid values of some tissues from cultured red sea bream and its dietary α-tocopherol levels. Nippon Suisan Gakkaishi 55: 1435–1439.
Nematipour, G.M., Brown, M.L. and Gatlin, D.M., III. 1992. Effects of dietary carbohydrate:lipid ratio on growth and body composition of hybrid striped bass. J. World Aquacult. Soc. 23: 128–132.
Nematipour, G.R. and Gatlin, D.M., III. 1993. Requirement of hybrid striped bass for dietary (n-3) highly unsaturated fatty acids. J. Nutr. 123:744–753.
NRC (National Research Council) 1993. Nutrient Requirements of Fish. National Academy Press, Washington, DC.
Rej, R. and Hørder, M. 1983. Aspartate aminotransferase. In: Methods in Enzymatic Analysis, Vol. III: Enzymes 1: Oxidoreductases, Transferases. pp. 416–433. Edited by H.U. Bergmeyer. Verlag Chemie, Deerfield Beach, FL.
Roem, A.J., Kohler, C.C. and Stickney, R.R. 1990. Vitamin E requirements of the blue tilapia, Oreochromis aureus (Steindachner), in relation to dietary lipid level. Aquaculture 87: 155–164.
SAS Institute, Inc. 1985. SAS User's Guide: Statistics, Version 5 Edition. Cary, NC. 956 pp.
Serrano, J.A., Nematipour, G.R. and Gatlin, D.M., III. 1992. Dietary protein requirement of the red drum (Sciaenops ocellatus) and the relative use of dietary carbohydrate and lipid. Aquaculture 101: 283–291.
Shavila, J., Ioannides, C., King, L.L. and Parke, D.V. 1994. Effect of high fat diet on liver microsomal oxygenation in ferret. Xenobiotica 24: 1063–1076.
Stéphan, G., Guillaume, J. and Lamour, F. 1995. Lipid peroxidation in turbot (Scophthalmus maximus) tissue: effect of dietary vitamin E and dietary n-6 or n-3 polyunsaturated fatty acids. Aquaculture 130: 251–268.
Thomas, P. and Boyd, N. 1988. Induced spawning of spotted seatrout, red drum and orangemouth corvina (family: Sciaenidae) with luteinizing hormone-releasing hormone analog injection. Contr. Marine Sci. 30: 43–48.
Thomas, P. and Wofford, H.W. 1993. Effects of cadmium and Aroclor 1254 on lipid peroxidation, glutathione peroxidase activity, and selected antioxidants in Atlantic croaker tissues. Aquat. Tox. 27: 159–178.
Tucker, J.W., Jr., Lellis, W.A., Vermeer, G.V., Roberts, D.E., Jr., and Woodward, P.N.1997. The effects of experimental starter diets with different levels of soybean or menhaden oil on red drum (Sciaenops ocellatus). Aquaculture 149: 323–339.
Wade, A.E., Bellows, J. and Dharwadkar, S. 1986. Influence of dietary menhaden oil on the enzymes metabolizing drugs and carcinogens. Drug Nutr. Interact. 4: 339–347.
Washburn, B.S., Beden, D.G., Gassman, N.J. and Walsh, P.J. 1994. Brevetoxin: tissue distribution and effect on cytochrome P450 enzymes in fish. Toxicon 32: 799–805.
Washburn, B.S., Vines, C.A., Baden, D.G., Hinton, D.E. and Walsh, P.J. 1996. Differentialeffects of brevetoxinis and β-naphthoflavone on xenobiotic metabolizing enzymes in striped bass (Morone saxatilis). Aquat. Tox. 35: 1–10.
Watanabe, T., Takeuchi, T., Wada, M. and Uehara, R. 1981. The relationship between dietary lipid levels and α-tocopherol requirement of rainbow trout. Bull. Jap. Soc. Sci. Fish. 47: 1463–1471.
Williams, C.D. and Robinson, E.H. 1988. Response of red drum to various dietary levels of menhaden oil. Aquaculture 70: 107–120.
Williams, D.E., Carpenter, H.M., Buhler, D.R., Kelly, J.D. and Dutchuk, M. 1992. Alterations in lipid peroxidation, antioxidant enzymes, and carcinogen metabolism in liver microsomes of vitamin E-deficient trout and rat. Toxicol. Appl. Pharmacol. 116: 78–84.
Wilson, R.P. and Poe, W.E. 1988. Choline nutrition of fingerling channel catfish. Aquaculture 68: 65–71.
Yasuda, S., Watanabe, S., Kobayashi, T. and Okuyama, H. 1997. Docosahexaenoic acid-rich fish oil does not enhance the elevation of serum transaminase and liver triacylglycerol induced by carbon tetrachloride in mice. Lipids 32: 1249–1255.
Yu, B.P. 1994. Cellular defenses against damage from reactive oxygen species. Physiol. Rev. 74: 139–162.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Craig, S., Washburn, B. & Gatlin, D. Effects of dietary lipids on body composition and liver function in juvenile red drum, Sciaenops ocellatus. Fish Physiology and Biochemistry 21, 249–255 (1999). https://doi.org/10.1023/A:1007843420128
Issue Date:
DOI: https://doi.org/10.1023/A:1007843420128