Abstract
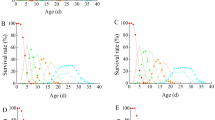
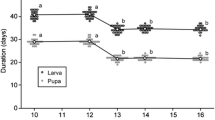
Growth responses to temperature and resource limitation in three dipteran species with similar life histories were compared. With respect to current life history theory, two points are raised. First, growth rate in real time increased steeply with temperature in all species, following the standard pattern. However, when expressed in physiological time growth rate increased as temperature decreased in the yellow dung fly Scathophaga stercoraria, remained approximately constant in Sepsis cynipsea, and increased in Drosophila melanogaster. These responses can be understood as adaptations to climate and seasonality. It is concluded that some patterns of adaptation may be more easily interpreted if, and some may even go undetected unless, they are analysed in physiological time. Second, a decrease in body size, development rate and growth rate when resources are limited is believed to be nearly universal and generally predicted by life history models. Despite their similar life histories, the three species investigated showed qualitatively different growth responses to larval food shortage. At unlimited resources, yellow dung flies showed the fastest initial larval body mass gain per unit time, while those of S. cynipsea and D. melanogaster were lower and about equal. The period of no body mass gain at the end of larval development was longest in S. stercoraria and shortest in S. cynipsea. When facing resource limitation, S. stercoraria emerged smaller but earlier (thus nearly maintaining their growth rate), S. cynipsea smaller after the same development period, and D. melanogaster smaller and later (showing reduced and much reduced growth, respectively). It is concluded that whether growth really slows when resources are limited depends on the precise ecological circumstances of the species in question. More refined models, particularly those where mortality costs are independent of time, and more experiments are necessary to account for the variation in growth and size and age at maturity present in nature.
Similar content being viewed by others
References
Abrams, P.A., Leimar, O., Nylin, S. and Wiklund, C. (1996) The effect of flexible growth rates on optimal sizes and development times in a seasonal environment. Am. Nat. 147, 381–395.
Anholt, B.R. and Werner, E.E. (1995) Interaction between food availability and predation mortality mediated by adaptive behavior. Ecology 76, 2230–2234.
Arnold, C.Y. (1959) The determination and significance of the base temperature in a linear heat unit system. J. Am. Soc. Hortic. Sci. 74, 430–445.
Arnold, C.Y. (1960) Maximum-minimum temperatures as a basis for computing heat units. J. Am. Soc. Hortic. Sci. 76, 682–692.
Atkinson, D. (1994) Temperature and organism size — A biological law for ectotherms? Adv. Ecol. Res. 25, 1–58.
Atkinson, D. (1995) Effects of temperature on the size of aquatic ectotherms: exceptions to the general rule. J. Therm. Biol. 20, 61–74.
Atkinson, D. and Sibly, R.M. (1997) Why are organisms usually bigger in colder environments? Making sense of a life history puzzle. Trends Ecol. Evol. 12, 235–239.
Bakker, K. (1959) Feeding period, growth, and pupation in larvae of Drosophila melanogaster. Ent. Exp. Appl. 2, 171–186.
Berrigan, D. and Charnov, E.L. (1994) Reaction norms for age and size at maturity in response to temperature: a puzzle for life historians. Oikos 70, 474–478.
Berrigan, D. and Koella, J.C. (1994) The evolution of reaction norms: simple models for age and size at maturity. J. Evol. Biol. 7, 549–566.
Blanckenhorn, W.U. (1994) Fitness consequences of alternative life histories in water striders, Aquarius remigis. Oecologia 97, 354–365.
Blanckenhorn, W.U. (1997a) Effects of temperature on growth, development and diapause in the yellow dung fly — against all the rules? Oecologia 111, 318–324.
Blanckenhorn, W.U. (1997b) Altitudinal life history variation in the dung flies Scathophaga stercoraria and Sepsis cynipsea. Oecologia 109, 342–352.
Blanckenhorn, W.U. (1998a) Adaptive phenotypic plasticity in growth, development, and diapause in the yellow dung fly. Evolution 52, 1394–1407.
Blanckenhorn, W.U. (1998b) Altitudinal differentiation in diapause response in two species of dung flies. Ecol. Entomol. 23, 1–8.
Blanckenhorn, W.U., Reusch, T. and Mühlhäuser, C. (1998) Fluctuating asymmetry, body size and sexual selection in the dung fly Sepsis cynipsea — testing the good genes assumptions and predictions. J. Evol. Biol. 11, 735–753.
Church, R.B. and Robertson, F.W. (1966) Biochemical analysis of genetic differences in the growth of Drosophila. Genet. Res. Camb. 7, 383–407.
Danks, H.V. (1987) Insect Dormancy: An Ecological Perspective. Biological Survey of Canada, Ottawa.
David, J. and Clavel, M.-F. (1967a) Influence de la température subie au cours du développement sur divers caractères biométriques des adultes de Drosophila melanogaster. J. Ins. Physiol. 13, 717–729.
David, J. and Clavel, M.-F. (1967b) Influence de la température d'élévage sur la mortalité larvonymphale et la durée de développement de la Drosophile. Nat. Can. 94, 209–219.
Eigenbrodt, H.J. (1930) The somatic effects of temperature on a homozygous race of Drosophila. Physiol. Zool. 3, 392–411.
Fox, C.W., Martin, J.D., Thakar, M.S. and Mousseau, T.A. (1996) Clutch size manipulations in two seed beetles: consequences for progeny fitness. Oecologia 108, 88–94.
Fraser, D.F. and Gilliam, J.F. (1992) Nonlethal impacts of predator invasion: facultative suppression of growth and reproduction. Ecology 73, 959–970.
Gilbert, P., Moreteau, B. Moreteau, J.-C. and David, J.R. (1996) Growth temperature and adult pigmentation in two Drosophila sibling species: an adaptive convergence of reaction norms in sympatric populations? Evolution 50, 2346–2353.
Janisch, E. (1925) Über die Temperaturabhängigkeit biologischer Vorgänge und ihre kurvenmässige Analyse. Pflügers Arch. Physiol. 209, 414–436.
Kawecki, T.J. and Stearns, S.C. (1993) The evolution of life histories in spatially heterogeneous environments: optimal reaction norms revisited. Evol. Ecol. 7, 155–174.
Kozlowski, J. (1992) Optimal allocation of resources to growth and reproduction: implications for age and size at maturity. Trends Ecol. Evol. 7, 15–19.
Kozlowski, J. and Wiegert, R.G. (1987) Optimal age and size at maturity in annuals and perennials with determinate growth. Evol. Ecol. 1, 231–244.
Morf, C. (1997) Saisonale, tageszeitliche und räumliche Variation der sexuellen Selektion bei der Schwingfliege Sepsis cynipsea. Diploma Thesis, University of Zürich.
Møller, H., Smith, R.H. and Sibly, R.M. (1989a) Evolutionary demography of a bruchid beetle. I. Quantitative genetical analysis of the female life history. Funct. Ecol. 3, 673–681.
Møller, H., Smith, R.H. and Sibly, R.M. (1989b) Evolutionary demography of a bruchid beetle. II. Physiological manipulations. Funct. Ecol. 3, 683–691.
Mühlhäuser, C., Blanckenhorn, W.U. and Ward, P.I. (1996) The genetic component of copula duration in the yellow dung fly. Anim. Behav. 51, 1401–1407.
Negus, N.C., Berger, P.J. and Pinter, A.J. (1992) Phenotypic plasticity of the montane vole (Microtus montanus) in unpredictable environments. Can. J. Zool. 68, 619–640.
Newman, R.A. (1992) Adaptive plasticity in amphibian metamorphosis. Bioscience 42, 671–678.
Nijhout, H.F. (1994) Insect Hormones. Princeton University Press, Princeton, NJ.
Nunney, L. (1996) The response to selection for fast larval development in Drosophila melanogaster and its effect on adult weight: an example of a fitness trade-off. Evolution 50, 1193–1204.
Nylin, S., Gotthard, K. and Wiklund, C. (1996) Reaction norms for age and size at maturity in Lasiommata butterflies: predictions and tests. Evolution 50, 1351–1358.
Prosser, C.L. (1973) Comparative Animal Physiology. W.B. Saunders Co, Philadelphia, PA.
Prowser, L. (1935) The effects of temperature on the durations of the developmental stages of Drosophila melanogaster. Physiol. Zool. 8, 474–520.
Ratte, H.T. (1985) Temperature and Insect development. In K.H. Hoffman (ed.) Environmental Physiology and Biochemistry of Insects, Springer, Heidelberg, pp. 31–66.
Robertson, F.W. (1963) The ecological genetics of growth in Drosophila. 6. The genetic correlation between the duration of the larval period and body size in relation to larval diet. Genet. Res. Camb. 4, 74–92.
Roff, D.A. (1984) The evolution of life history parameters in teleosts. Can. J. Fish. Aquat. Sci. 41, 989–1000.
Roff, D.A. (1986) Predicting body size with life history models. Bioscience 36, 316–323.
Roff, D.A. (1992) The Evolution of Life Histories: Theory and Analysis. Chapman and Hall, New York, NY.
Rowe, L. and Ludwig, D. (1991) Size and timing of metamorphosis in complex life cycles: time constraints and variation. Ecology 72, 413–427.
Schmidt-Nielsen, K. (1983) Animal Physiology: Adaptation and Environment, 3rd ed. Cambridge University Press, Cambridge.
Schulz, K. (1989) Vergleichende Untersuchung zur Biologie einiger kuhfladenbewohnender Arten der Gattung Sepsis (Diptera, Sepsidae). Diploma thesis, Free University Berlin.
Shorrocks, B. (1970) Population fluctuations in the fruit fly (Drosophila melanogaster) maintained in the laboratory. J. Anim. Ecol. 39, 229–253.
Sibly, R.M. and Atkinson, D. (1994) How rearing temperature affects optimal adult size in ectotherms. Funct. Ecol. 8, 486–493.
Sokal, R.R. and Rohlf, F.J. (1995) Biometry. Freeman and Co, New York, NY.
Stearns, S.C. (1992) The Evolution of Life Histories. Oxford University Press, Oxford.
Stearns, S.C. and Koella, J. (1986) The evolution of phenotypic plasticity in life history traits: predictions of reaction norms for age and size at maturity. Evolution 40, 893–914.
Sweeney, B.W. and Vannote, R.L. (1978) Size variation and the distribution of hemimetabolous aquatic insects: two thermal equilibrium hypotheses. Science 200, 444–446.
Tabachnick, B.G. and Fidell, L.S. (1989) Using Multivariate Statistics. Harper and Row, New York, NY.
Taylor, F. (1981) Ecology and evolution of physiological time in insects. Am. Nat. 117, 1–23.
van der Have, T.M. and de Jong, G. (1996) Adult size in ectotherms: temperature effects on growth and differentiation. J. Theor. Biol. 183, 329–340.
van Straalen, N.M. (1983) Physiological time and time-invariance. J. Theor. Biol. 104, 349–357.
Visser, M.E. (1994) The importance of being large: the relationship between size and fitness in females of the parasitoid Aphaereta minuta (Hymenoptera: Braconidae). J. Anim. Ecol. 63, 963–978.
Wang, J.Y. (1960) A critique of the heat unit approach to plant response studies. Ecology 41, 785–790.
Wigglesworth, V.B. (1972) The Principles of Insect Physiology, 7th edn. Chapman and Hall, London.
Wilbur, H.M. and Collins, J.P. (1973) Ecological aspects of amphibian metamorphosis. Science 182, 1305–1314.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Blanckenhorn, W.U. Different growth responses to temperature and resource limitation in three fly species with similar life histories. Evolutionary Ecology 13, 395–409 (1999). https://doi.org/10.1023/A:1006741222586
Issue Date:
DOI: https://doi.org/10.1023/A:1006741222586