Abstract
In the era of nanotechnology, nanoparticles (NPs) of metals and metal oxides/chalcogenides are widely been used in medical applications where antibiotic-resistant microorganisms become a serious threat to the human health. Cobalt ferrite (CoFe2O4) NPs, synthesized by a simple and cost-effective sol–gel auto-combustion method are envisaged for in vitro antimicrobial activities against Gram-positive bacteria (Bacillus subtilis; Staphylococcus aureus) and Gram-negative bacteria (Escherichia coli; Pseudomonas aeruginosa). The structure, morphology, elemental analyses and surface area of CoFe2O4 NPs are initially screened. The antimicrobial efficiency of CoFe2O4 NPs is found to be optimum against the Gram-negative bacteria Escherichia coli (15 mm). In addition, membrane leakage assays performed to evaluate the intracellular cytoplasmic leakage with CoFe2O4 NPs demonstrate the ability to destroy the bacterial membrane integrity, confirming their antimicrobial potential.
Similar content being viewed by others
Introduction
In post-antibiotic era, low antibiotic susceptibility of pathogenic bacteria is still continuing worrisome situation. Both Gram-positive and Gram-negative bacterial strains are found to have adverse impacts on human as well as on animal lives; demand new alternative approach [1]. Nanotechnology is a great field to deal with diverse aspects of eco-system [2, 3]. Nanoparticles (NPs) of metal, metal oxide, polymer and carbonaceous materials offer a wide range of properties followed by applications which allowed researchers to cross the boundaries of specialized area studies than known prevalent one [4, 5]. Till date, several NPs have been envisaged for their efficacy in water purification, medicine and food industry [6,7,8]. The CoFe2O4 is one of the most important nanoparticles which has a wide energy bandgap, semiconductor in nature and electrocatalytic in signature which makes it a suitable material in energy harvesting/storage and conversion [9, 10], chemoresistive sensor [11], dye degradation [12] and pathogen detection [13]. Moreover, its magnetic property has attracted considerable attention in biomedical applications such as drug delivery, tissue repair, magnetic resonance imaging and magnetic fluid hyperthermia [14, 15]. Recently, a size and shape-dependent bactericidal properties of NPs have been explored [16, 17] which has endowed an increasing interest in the synthesis method of NPs as it plays a crucial role in determining the physico-chemical properties of NPs. Several synthesis methods such as co-precipitation [18], hydrothermal [19], micro-emulsion [20], spray pyrolysis [21], and sol–gel auto-combustion [22], etc., have successfully been addressed in the literature for developing ferrites of different structures and morphologies. Among these, sol–gel auto-combustion method is found to be more suitable for the fabrication of ferrite NPs on a mass scale with desired sizes and shapes. This method is quite simple, rapid and economic which offers good chemical homogeneity with low-external energy consumption [23, 24]. Using this method, the size, surface area and the morphology of the obtained ferrites can be engineered by changing the annealing temperature and synthesis conditions [25, 26]. On reducing the particle size, an improved antimicrobial efficacy of CoFe2O4 NPs was reported by Žalnėravičius et al. [27]. However, this investigation is limited to fungal strain. With reference to the studies carried out so far, it is identified that cobalt ferrites exhibits antimicrobial activity [28, 29]. Although as on date, their bactericidal effect is not clearly concluded. To target the membrane integrity of microorganisms is a crucial strategy to combat with pathogens [30]. Thereby, targeting membrane integrity will be interesting to understand the mechanism of action of cobalt ferrites.
On the basis of above experimentation history, present work deals with the synthesis and antibacterial activity measurements of CoFe2O4 NPs. In first stage, CoFe2O4 NPs were synthesized by a cost-effective sol–gel auto-combustion method, air-annealed at 500 °C for 5 h and characterized for their structure, morphology and surface area measurements. These CoFe2O4 NPs were applied in antibacterial efficiency on selected pathogen using agar well-diffusion technique and studied for membrane integrity by membrane leakage assay.
Materials
Selected pathogenic strains Gram-positive bacteria Bacillus subtilis (MTCC-441) and Staphylococcus aureus (MTCC-3160), and Gram-negative bacteria Escherichia coli (MTCC-40) and Pseudomonas aeruginosa (MTCC-424) were purchased from the Institute of Microbial Technology (IMTECH), Chandigarh, India.
Synthesis and measurements
Synthesis of CoFe2O4 NPs
The NPs of CoFe2O4 were synthesized from cobalt nitrate [Co (NO3)2·6H2O] and ferric nitrate [Fe(NO3)3·9H2O] with addition of citric acid as reducing agent by sol–gel auto-combustion method, following the protocol described by Gore et al. [22]. Stoichiometric amounts of said chemicals were added into distilled water and pH of the solution was adjusted to 7 using ammonia solution. This solution was then magnetically stirred for ~ 3 h at 80–90 °C until a wet gel of the metal nitrates was formed. The obtained gel was allowed to burn until it turned out to be an ash. Finally, this ash was annealed at 500 °C for 5 h and grinded in mortar and pestle to form the powder of CoFe2O4 NPs before their implication.
Characterizations
The X-ray diffraction pattern of CoFe2O4 NPs was recorded on X-ray diffractometer (XRD, D8-Discovery Bruker, Cu Kα, 40 kV, 40 mA) which was scanned from 10 to 100°. Scanning electron microscopy (SEM, Hitachi, S-4800, 15 kV) digital surface image was recorded to confirm the surface appearance of these NPs. Elemental composition analysis of the CoFe2O4 NPs was performed using energy-dispersive X-ray spectroscopy (EDX) for knowing the surface elements followed their quantitative contributions. The Brunauer–Emmett–Teller (BET) measurement was obtained using a Micrometrics ASAP2010 analyser to study the surface area of the CoFe2O4 NPs.
Antimicrobial study
Antimicrobial activity of CoFe2O4 NPs was determined by agar well-diffusion method [31]. Briefly, overnight grown culture of test organisms was used to prepare standards inoculums by adjusting turbidity equal to the standard 0.5 McFarland solution at 600 nm. About 50 μL inoculum suspension of organisms was swabbed uniformly on Mueller–Hinton agar plate. In each agar plate, well of 6 mm diameter was made by flame-sterilized cork borer. Using a micropipette, 100 μL solution of the CoFe2O4 NPs was poured in each well of all the plates and all plates were kept for overnight incubation at 37 °C. The incubated plates were examined for antimicrobial activity.
Bacterial membrane leakage assay
To find out the membrane damage, membrane leakage assay was studied in the presence and absence of CoFe2O4 NPs as reported earlier by Li et al. [32] with a little modification. The membrane leakage assay was performed by estimating the amount of reducing sugars and proteins from test bacterial membrane. In this assay different volume of Mueller–Hinton broth, NPs, and test pathogens were added into 10 mL culture with final concentration of 100 μg/mL CoFe2O4 NPs and 109 cfu/mL test pathogens. This culture was incubated at 37 °C with shaking at 150 rpm. After 2 and 24 h incubation, 1 mL culture was sampled for sugar estimation whereas, protein estimation was done at different time intervals viz. 2, 4, 6 and 8 h, centrifuged at 12,000 rpm to remove the bacteria cell, collected supernatant was frozen at 4 °C immediately and then the concentrations of reducing sugars and proteins were determined by Bradford and Miller standard methods [33, 34]. Control experiments were also carried out without NPs.
Results and discussion
Structure and morphology analyses
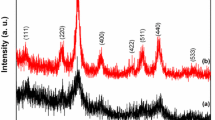
The XRD peaks of various intensities shown in Fig. 1a were in accordance to (111), (220), (311), (400), (422), (511), (440), (533) and (731) reflection planes of Joint Committee on Powder Diffraction Standards (JCPDS) index card 22-1086, supporting for the formation of polycrystalline CoFe2O4. The average crystallite size calculated by Debey–Scherrer equation, \(D = \frac{K\lambda }{\beta \cos \theta } ,\) where ‘D’ is the crystallite size, ‘λ’ is the X-ray wavelength (1.5406 Å), ‘β’ is the full width at half maximum (FWHM) for (311) and ‘θ’ is the diffraction angle, was ~ 28 nm.
a XRD pattern, b SEM micrograph and c EDX elemental analysis of the CoFe2O4 NPs [43]
The SEM image of CoFe2O4 NPs is shown in Fig. 1b, confirming the random distribution of NPs in an aggregated form due to which it was complicated to find out the precise size and shape of individual NPs. Figure 1c shows the EDX elemental analysis of the CoFe2O4 NPs where 13.80, 27.68 and 58.52% atomic compositions of the Co, Fe and O elements were in good agreement to 1:2:4 ratios so as to obtain the chemical stoichiometry of CoFe2O4.
Surface area and pore-size measurements
The surface area of CoFe2O4 NPs was obtained using the standard multi-point BET method and is shown in Fig. 2. The surface area of CoFe2O4 NPs was 22.15 m2/g. The average pore diameter of CoFe2O4 NPs (inset of Fig. 2), obtained from Barrett–Joyner–Halenda (BJH) method, was 110 nm. The porosity of the nanomaterials is an important property that was also been studied. Microporous, mesoporous and macroporous nanomaterials are commonly utilized for biomedical applications because of their high surface area which helps to improve the absorbent and adsorbent properties of the materials and thereby enhance the cellular adhesion [35]. The selection of such materials mainly depends upon the type of the applications such as antibacterial activity, drug delivery, enzyme immobilization, medical imaging and tissue engineering, etc. [36]. As-obtained CoFe2O4 NPs demonstrated macroporous signature as pore diameter was > 50 nm which would be beneficial for better antibacterial activities. The present results were analogous to Naikoo et al. findings who reported good antibacterial activity of the silver monoliths on account of their macroporous character and high surface free energy [37].
Antimicrobial efficacy of CoFe2O4 NPs
Auto-combusted CoFe2O4 NPs were envisaged in antimicrobial activity and results are expressed as a zone of inhibition (mm) in Fig. 3. The obtained data confirmed a noticeable antimicrobial activity at 500 μg/mL concentration of CoFe2O4 NPs against both Gram-positive and Gram-negative bacteria, which is summarized in Table 1. The maximum zone of inhibition was recorded for the E. coli (15 ± 0.30 mm) and a least zone of inhibition was recorded for S. aureus (09 ± 0.60 mm). Results of present study demonstrate better antimicrobial activity at lower concentration than the previously reported by Kooti et al. [28]. This may be due to the particle size difference while both studies show that Gram-positive bacteria are less susceptible towards CoFe2O4 NPs. This effect attributed to difference in the structural and chemical composition of Gram-positive and Gram-negative bacteria cell walls, in accordance to previous findings by Sanpo et al. [29], which also suggests the CoFe2O4 NPs were comparatively less active against the Gram-positive bacteria.
Cell membrane integrity towards CoFe2O4 NPs
The NPs can disturb the cell membrane of pathogenic bacteria by resulting in cytoplasmic material leakage through cell membrane [38,39,40]. Thereby, the membrane leakage assay was performed to understand effect of CoFe2O4 NPs on the bacterial cell membrane. An effect of CoFe2O4 NPs on the membrane leakage of reducing sugars and proteins is shown in Fig. 4a, b, respectively. In this assay, reducing sugars were estimated after exposure of CoFe2O4 NPs for 2 h and 24 h, this time interval showed a notable increment in amount of leakage sugar. Similarly, leakage of proteins was estimated after 2 h and 4 h, where the amount of leakage protein increased. After this time, decrease in the amount of protein content evidenced. Significant differences were observed in proteins released from the bacteria at different point of times, due to the proteolytic enzyme responsible for protein degradation, which could be released after membrane damage [41]. Both reducing sugar and protein amount was observed to be optimum in E. coli followed by P. aeruginosa, B. subtilis and S. aureus.
Figure 5 portrays the plausible mechanism of antimicrobial action of CoFe2O4 NPs. Moreover, these findings suggest that CoFe2O4 NPs hold potential to kill both Gram-positive and Gram-negative bacteria because both are negatively charged cells which favour electrostatic interaction with NPs or released ions from them [30]. Even though the Gram-negative bacteria are more sensitive towards CoFe2O4 NPs, reason is that the Gram-negative bacteria differ in their structural and chemical constituents which are surrounded by outer layer of lipopolysaccharides, whereas, Gram-positive bacteria are covered by peptidoglycan. Lipopolysaccharide is less rigid as compared to peptidoglycan which can easily break [29]. However, the exact mechanism behind antimicrobial activity of CoFe2O4 NPs is not known. Experimental results of present study corroborate that the CoFe2O4 NPs can cause for intracellular material leakage from all tested bacteria. Therefore, we speculate the antimicrobial mechanism of ferrites involved in the production of free radicals mainly reactive oxygen species (ROS) via Fenton reaction [42] and generates oxidative stress on bacteria which causes the breakage of cell membrane with leakage of cytoplasmic materials (sugar and protein), leads to loss of metabolic activity resulting in cell death. This study is first of its kind to examine the membrane leakage by CoFe2O4 NPs.
Conclusion
In this study, polycrystalline CoFe2O4 NPs were synthesized using a cost-effective sol–gel auto-combustion method. Macroporous CoFe2O4 NPs (with pore diameter of 110 nm) have demonstrated 22.15 m2/g surface area which may facilitate better interaction with bacterial cells and eventually causes the cell membrane damage. Cytoplasmic leakage confirms the membrane disintegration that leads to loss of essential bioenergetic functions associated with cell death. Furthermore, the CoFe2O4 NPs shows a good antimicrobial activity against all tested bacteria, especially, Gram-negative bacteria. Present study revealed that the CoFe2O4 NPs are a potential antimicrobial agent and can be utilized in various antimicrobial applications as well as it can be a new opening for other ferrites and composite structures, a work of future studies.
References
Davies, J., Davies, D.: Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 74(3), 417–433 (2010)
Bhatia, D., Mittal, A., Malik, D.K.: Antimicrobial activity of PVP coated silver nanoparticles synthesized by Lysinibacillus varians. Biotech 6(2), 196 (2016)
Liu, J., Qiao, S.Z., Hu, Q.H.: Magnetic nanocomposites with mesoporous structures: synthesis and applications. Small 7(4), 425–443 (2011)
Kudr, J., Haddad, Y., Richtera, L., Heger, Z., Cernak, M., Adam, V., Zitka, O.: Magnetic nanoparticles: from design and synthesis to real world applications. Nanomaterials 7(9), 243 (2017)
Mirza, A.Z., Siddiqui, F.A.: Nanomedicine and drug delivery: a mini review. Int. Nano Lett. 4(1), 94 (2014)
Bora, T., Dutta, J.: Applications of nanotechnology in wastewater treatment—a review. J. Nanosci. Nanotechnol. 14(1), 613–626 (2014)
Salata, O.V.: Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2(1), 3 (2004)
Chellaram, C., Murugaboopathi, G., John, A.A., Sivakumar, R., Ganesan, S., Krithika, S., Priya, G.: Significance of nanotechnology in food industry. APCBEE Proc. 8, 109–113 (2014)
Allaedini, G., Tasirin, S.M., Aminayi, P.: Magnetic properties of cobalt ferrite synthesized by hydrothermal method. Int. Nano Lett. 5(4), 183–186 (2015)
Kasapoglu, N., Birsöz, B., Baykal, A., Köseoglu, Y., Toprak, M.: Synthesis and magnetic properties of octahedral ferrite NiχCo1−χFe2O4 nanocrystals. Open Chem. 5(2), 570–580 (2007)
Srivastava, R., Yadav, B.C.: Ferrite materials: introduction, synthesis techniques, and applications as sensors. Int. J. Green Nanotechnol. 4(2), 141–154 (2012)
Sharma, R., Singhal, S.: Photodegradation of textile dye using magnetically recyclable heterogeneous spinel ferrites. J. Chem. Technol. Biotechnol. 90(5), 955–962 (2015)
Bohara, R.A., Throat, N.D., Mulla, N.A., Pawar, S.H.: Surface-modified cobalt ferrite nanoparticles for rapid capture, detection, and removal of pathogens: a potential material for water purification. Appl. Biochem. Biotechnol. 182(2), 598–608 (2017)
Sanpo, N., Tharajak, J., Li, Y., Berndt, C.C., Wen, C., Wang, J.: Biocompatibility of transition metal-substituted cobalt ferrite nanoparticles. J. Nanopart. Res. 16(7), 2510 (2014)
Gharibshahian, M., Mirzaee, O., Nourbakhsh, M.S.: Evaluation of superparamagnetic and biocompatible properties of mesoporous silica coated cobalt ferrite nanoparticles synthesized via microwave modified Pechini method. J. Magn. Magn. Mater. 425, 48–56 (2017)
Lu, Z., Rong, K., Li, J., Yang, H., Chen, R.: Size-dependent antibacterial activities of silver nanoparticles against oral anaerobic pathogenic bacteria. J. Mater. Sci. Mater. Med. 24(6), 1465–1471 (2013)
Pal, S., Tak, Y.K., Song, J.M.: Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 73(6), 1712–1720 (2007)
Kim, Y.I., Kim, D., Lee, C.S.: Synthesis and characterization of CoFe2O4 magnetic nanoparticles prepared by temperature-controlled coprecipitation method. Phys. B 337(1–4), 42–51 (2003)
Zhao, L., Zhang, H., Xing, Y., Song, S., Yu, S., Shi, W., Guo, X., Yang, J., Lei, Y., Cao, F.: Studies on the magnetism of cobalt ferrite nanocrystals synthesized by hydrothermal method. J. Solid State Chem. 181(2), 245–252 (2008)
Khan, M.A., Sabir, M., Mahmood, A., Asghar, M., Mahmood, K., Khan, M.A., Ahmad, I., Sher, M., Warsi, M.F.: High frequency dielectric response and magnetic studies of Zn1−xTbxFe2O4 nanocrystalline ferrites synthesized via micro-emulsion technique. J. Magn. Magn. Mater. 360, 188–192 (2014)
Takayama, A., Okuya, M., Kaneko, S.: Spray pyrolysis deposition of NiZn ferrite thin films. Solid State Ionics 172(1–4), 257–260 (2004)
Gore, S.K., Jadhav, S.S., Jadhav, V.V., Patange, S.M., Naushad, M., Mane, R.S., Kim, K.H.: The structural and magnetic properties of dual phase cobalt ferrite. Sci. Rep. 7(1), 2524 (2017)
Costa, A.C.F., Morelli, M.R., Kiminami, R.H.: Microstructure and magnetic properties of Ni1−xZnxFe2O4 synthesized by combustion reaction. J. Mater. Sci. 42(3), 779–783 (2007)
Sutka, A., Mezinskis, G.: Sol-gel auto-combustion synthesis of spinel-type ferrite nanomaterials. Front. Mater. Sci. 6(2), 128–141 (2012)
Toksha, B.G., Shirsath, S.E., Patange, S.M., Jadhav, K.M.: Structural investigations and magnetic properties of cobalt ferrite nanoparticles prepared by sol–gel auto combustion method. Solid State Commun. 147(11–12), 479–483 (2008)
Zak, A.K., Abrishami, M.E., Majid, W.A., Yousefi, R., Hosseini, S.M.: Effects of annealing temperature on some structural and optical properties of ZnO nanoparticles prepared by a modified sol–gel combustion method. Ceram. Int. 37(1), 393–398 (2011)
Žalnėravičius, R., Paškevičius, A., Kurtinaitiene, M., Jagminas, A.: Size-dependent antimicrobial properties of the cobalt ferrite nanoparticles. J. Nanopart. Res. 18(10), 300 (2016)
Kooti, M., Saiahi, S., Motamedi, H.: Fabrication of silver-coated cobalt ferrite nanocomposite and the study of its antibacterial activity. J. Magn. Magn. Mater. 333, 138–143 (2013)
Sanpo, N., Wen, C., Berndt, C.C., Wang, J.: Antibacterial properties of spinel ferrite nanoparticles. Microbial pathogens and strategies for combating them: science, technology and education. Spain: Formatex Research Centre, 239–250 (2013)
Slavin, Y.N., Asnis, J., Häfeli, U.O., Bach, H.: Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 15(1), 65 (2017)
Balouiri, M., Sadiki, M., Ibnsouda, S.K.: Methods for in vitro evaluating antimicrobial activity: a review. J. Pharmaceut. Anal. 6(2), 71–79 (2016)
Li, W.R., Xie, X.B., Shi, Q.S., Zeng, H.Y., You-Sheng, O.Y., Chen, Y.B.: Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 85(4), 1115–1122 (2010)
Bradford, M.M.: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72(1–2), 248–254 (1976)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31(3), 426–428 (1959)
Cai, R., Wang, H., Cao, M., Hao, L., Zhai, L., Jiang, S., Li, X.: Synthesis and antimicrobial activity of mesoporous hydroxylapatite/zinc oxide nanofibers. Mater. Des. 87, 17–24 (2015)
Solano-Umaña, V., Vega-Baudrit, J.R.: Micro, Meso and Macro Porous Materials on Medicine. J. Biomater. Nanobiotechnol. 6(4), 247 (2015)
Naikoo, G.A., Thomas, M., Ganaie, M.A., Sheikh, M.U.D., Bano, M., Hassan, I.U., Khan, F.: Hierarchically macroporous silver monoliths using Pluronic F127: facile synthesis, characterization and its application as an efficient biomaterial for pathogens. J. Saudi Chem. Soc. 20(2), 237–244 (2016)
Beyth N., Houri-Haddad, Y., Domb, A., Khan, W., Hazan, R.: Alternative antimicrobial approach: nano-antimicrobial materials. Evid. Based Complem. Altern. Med. 2015 (2015)
Jung, W.K., Koo, H.C., Kim, K.W., Shin, S., Kim, S.H., Park, Y.H.: Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 74(7), 2171–2178 (2008)
Sondi, I., Salopek-Sondi, B.: Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 275(1), 177–182 (2004)
Davies, K.J., Lin, S.W.: Oxidatively denaturated proteins are degraded by an ATP-independent proteolytic pathway in Escherichia coli. Free Radical Biol. Med. 5(4), 225–236 (1988)
Casbeer, E., Sharma, V.K., Li, X.Z.: Synthesis and photocatalytic activity of ferrites under visible light: a review. Sep. Purif. Technol. 87, 1–14 (2012)
Raut, S.D., Awasarmol, V.V., Ghule, B.G., Shaikh, S.F., Gore, S., Sharma, R.P., Pawar, P.P., Mane, R.S.: Enhancement in room-temperature ammonia sensor activity of size-reduced cobalt ferrite nanoparticles on γ-irradiation. Mater. Res. Express 5, 065035 (2018)
Acknowledgements
The authors would like to thank the Director of UGC-DAE-CSR for allowing the EDAX facility.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sharma, R.P., Raut, S.D., Mulani, R.M. et al. Sol–gel auto-combustion mediated cobalt ferrite nanoparticles: a potential material for antimicrobial applications. Int Nano Lett 9, 141–147 (2019). https://doi.org/10.1007/s40089-019-0268-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40089-019-0268-4