Abstract
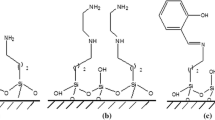
The authors describe the preparation of two kinds of periodic mesoporous organosilicas (PMOs). The first kind is monofunctional and has a bridged alkyl imidazolium framework (PMO-IL). The other is a two-dimensional (2D) hexagonal bifunctional periodic mesoporous organosilica (BFPMO) with bridged IL-phenyl or -ethyl units. The CPMOs were utilized as highly sensitive and stable sorbents for microextraction by packed sorbent. The materials were characterized by SEM, TEM, FT-IR, and N2 adsorption–desorption analysis. The adsorption capacities of the sorbents were investigated by using phenoxy acid herbicides as model analytes. The effects of bifunctionality and type of additional surface groups (phenyl or ethyl) on the efficiency of the extraction is emphasized. Three kinds of environmental contaminants, viz. phenoxy acid herbicides (CPAs), polycyclic aromatic hydrocarbons and chlorophenols were then studied with respect to their extraction by the sorbents. The interactions between the CPAs and the sorbents were evaluated by pH-changing processes to explore the interactions that play a major role. The selectivity of the sorbents was investigated by extraction of other types of analytes of with various polarity and charge. The BFPMOs display the typical good chemical stability of silica materials. The extraction properties are much better compared to commercial silicas. This is assumed to be due to the highly ordered mesoporous structures and the different types of probable interactions with analytes. The performance of the method was evaluated by extraction of CPAs as model analytes from aqueous samples, and quantification by GC with FID detection. Under optimized conditions, low limits of detection (0.1–0.5 μg.L−1) and a wide linearity (0.5–200 μg.L−1) were obtained. The method was applied to the trace analysis of CPAs in farm waters and rice samples.

Monofunctional periodic mesoporous organosilica with bridged alkyl imidazolium frameworks and bi-functional periodic mesoporous organosilica containing bridged ionic liquids and phenyl or -ethyl, have been successfully synthesized and utilized in microextractions by packed sorbent sorbents.




Similar content being viewed by others
References
Abdel-Rehim M (2004) New trend in sample preparation: on-line microextraction in packed syringe for liquid and gas chromatography applications: I. Determination of local anaesthetics in human plasma samples using gas chromatography–mass spectrometry. J Chromatogr B 801:317–321. https://doi.org/10.1016/j.jchromb.2003.11.042
Abdel-Rehim M (2010) Recent advances in microextraction by packed sorbent for bioanalysis. J Chromatogr A 1217:2569–2580. https://doi.org/10.1016/j.chroma.2009.09.053
Yang L, Said R, Abdel-Rehim M (2017) Sorbent, device, matrix and application in microextraction by packed sorbent (MEPS): a review. J Chromatogr B 1043:33–43. https://doi.org/10.1016/j.jchromb.2016.10.044
Moein MM, Abdel-Rehim A, Abdel-Rehim M (2015) Microextraction by packed sorbent (MEPS). TrAC Trends Anal Chem 67:34–44. https://doi.org/10.1016/j.trac.2014.12.003
Klimowska A, Wielgomas B (2018) Off-line microextraction by packed sorbent combined with on solid support derivatization and GC-MS: application for the analysis of five pyrethroid metabolites in urine samples. Talanta 176:165–171. https://doi.org/10.1016/j.talanta.2017.08.011
Montesano C, Simeoni MC, Curini R, Sergi M, Sterzo CL, Compagnone D (2015) Determination of illicit drugs and metabolites in oral fluid by microextraction on packed sorbent coupled with LC-MS/MS. Anal Bioanal Chem 407:3647–3658. https://doi.org/10.1007/s00216-015-8583-8
Matysik S, Matysik FM (2009) Microextraction by packed sorbent coupled with gas chromatography—mass spectrometry: application to the determination of metabolites of monoterpenes in small volumes of human urine. Microchim Acta 166:109–114. https://link.springer.com/article/. https://doi.org/10.1007/s00604-009-0170-2
Prieto A, Schrader S, Bauer C, Möder M (2011) Synthesis of a molecularly imprinted polymer and its application for microextraction by packed sorbent for the determination of fluoroquinolone related compounds in water. Anal Chim Acta 685:146–152. https://doi.org/10.1016/j.aca.2010.11.038
De Souza ID, Domingues DS, Queiroz ME (2015) Hybrid silica monolith for microextraction by packed sorbent to determine drugs from plasma samples by liquid chromatography–tandem mass spectrometry. Talanta 140:166–175. https://doi.org/10.1016/j.talanta.2015.03.032
Bagheri H, Alipour N, Ayazi Z (2012) Multiresidue determination of pesticides from aquatic media using polyaniline nanowires network as highly efficient sorbent for microextraction in packed syringe. Anal Chim Acta 740:43–49. https://doi.org/10.1016/j.aca.2012.06.026
Bagheri H, Banihashemi S, Zandian FK (2016) Microextraction of antidepressant drugs into syringes packed with a nanocomposite consisting of polydopamine, silver nanoparticles and polypyrrole. Microchim Acta 183:195–202. https://link.springer.com/article/. https://doi.org/10.1007/s00604-015-1606-5
Karimi B, Gholinejad M, Khorasani M (2012) Highly efficient three-component coupling reaction catalyzed by gold nanoparticles supported on periodic mesoporous organosilica with ionic liquid framework. Chem Commun 48:8961–8963. https://doi.org/10.1039/C2CC33320A
Karimi B, Elhamifar D, Yari O, Khorasani M, Vali H, Clark JH, Hunt AJ (2012) Synthesis and characterization of Aalkyl-imidazolium-based periodic mesoporous organosilicas: a versatile host for the immobilization of perruthenate (RuO4−) in the aerobic oxidation of alcohols. Chem Eur J 18:13520–13530. https://doi.org/10.1002/chem.201200380
Vathyam R, Wondimu E, Das S, Zhang C, Hayes S, Tao Z, Asefa T (2011) Improving the adsorption and release capacity of organic-functionalized mesoporous materials to drug molecules with temperature and synthetic methods. J Phys Chem C 115:13135–13150. https://doi.org/10.1021/jp1108587
Abolghasemi MM, Karimi B, Yousefi V (2013) Periodic mesoporous organosilica with ionic liquid framework as a novel fiber coating for headspace solid-phase microextraction of polycyclic aromatic hydrocarbons. Anal Chim Acta 804:280–286. https://doi.org/10.1016/j.aca.2013.10.022
Van Der Voort P, Esquivel D, De Canck E, Goethals F, Van Driessche I, Romero-Salguero FJ (2013) Periodic mesoporous organosilicas: from simple to complex bridges; a comprehensive overview of functions, morphologies and applications. Chem Soc Rev 42:3913–3955. https://doi.org/10.1039/C2CS35222B
Hoffmann F, Cornelius M, Morell J, Fröba M (2006) Silica-based mesoporous organic–inorganic hybrid materials. Angew Chem Int Ed 45:3216–3251. https://doi.org/10.1002/anie.200503075
Hunks WJ, Ozin GA (2005) Challenges and advances in the chemistry of periodic mesoporous organosilicas (PMOs). J Mater Chem 15:3716–3724. https://doi.org/10.1039/B504511H
Dral AP, Lievens C, ten Elshof JE (2017) Influence of monomer connectivity, network flexibility, and hydrophobicity on the hydrothermal stability of organosilicas. Langmuir 33:5527–5536. https://doi.org/10.1021/acs.langmuir.7b00971
Fujita S, Inagaki S (2008) Self-organization of organosilica solids with molecular-scale and mesoscale periodicities. Chem Mater 20:891–908. https://doi.org/10.1021/cm702271v
Olkhovyk O, Jaroniec M (2005) Periodic mesoporous organosilica with large heterocyclic bridging groups. J Am Chem Soc 127:60–61. https://doi.org/10.1021/ja043941a
Karimi B, Elhamifar D, Clark JH, Hunt AJ (2010) Ordered mesoporous Organosilica with ionic-liquid framework: an efficient and reusable support for the palladium-catalyzed Suzuki–Miyaura coupling reaction in water. Chem Eur J 16:8047–8053. https://doi.org/10.1002/chem.201000538
Karimi B, Elhamifar D, Clark JH, Hunt AJ (2011) Palladium containing periodic mesoporous organosilica with imidazolium framework (Pd@ PMO-IL): an efficient and recyclable catalyst for the aerobic oxidation of alcohols. Org Biomol Chem 9:7420–7426. https://doi.org/10.1039/C1OB05752A
Karimi B, Khorasani M, Vali H, Luque R (2015) Control of plugging in bifunctional periodic mesoporous organosilica with imidazolium framework (BFPMO) via stepwise addition of silica precursors. J Mater Chem A 3:6575–6585. https://doi.org/10.1039/C4TA06542E
Karimi B, Khorasani M, Vali H, Vargas C, Luque R (2015) Palladium nanoparticles supported in the nanospaces of imidazolium-based bifunctional PMOs: the role of plugs in selectivity changeover in aerobic oxidation of alcohols. ACS Catal 5:4189–4200. https://doi.org/10.1021/acscatal.5b00237
Amiri A, Saadati-Moshtaghin HR, Zonoz FM (2018) A hybrid material composed of a polyoxometalate of type BeW 12 O 40 and an ionic liquid immobilized onto magnetic nanoparticles as a sorbent for the extraction of organophosphorus pesticides prior to their determination by gas chromatography. Microchim Acta 185:176. https://doi.org/10.1007/s00604-018-2713-x
Gu W, Zhu X (2017) Nanoparticles of type Fe 3 O 4-SiO 2-graphene oxide and coated with an amino acid-derived ionic liquid for extraction of Al (III), Cr (III), cu (II), Pb (II) prior to their determination by ICP-OES. Microchim Acta 184:4279–4286. https://doi.org/10.1007/s00604-017-2469-8
Mousavi KZ, Yamini Y, Seidi S (2018) Dispersive liquid–liquid microextraction using magnetic room temperature ionic liquid for extraction of ultra-trace amounts of parabens. New J Chem 42:9735–9743. https://doi.org/10.1039/C8NJ01154K
Temtsin G, Asefa T, Bittner S, Ozin GA (2001) Aromatic PMOs: tolyl, xylyl and dimethoxyphenyl groups integrated within the channel walls of hexagonal mesoporous silicas. J Mater Chem 11:3202–3206. https://doi.org/10.1039/B103960C
Burleigh MC, Markowitz MA, Spector MS, Gaber BP (2001) Direct synthesis of periodic mesoporous organosilicas: functional incorporation by co-condensation with organosilanes. J Phys Chem B 105:9935–9942. https://doi.org/10.1021/jp011814k
Burleigh MC, Markowitz MA, Jayasundera S, Spector MS, Thomas CW, Gaber BP (2003) Mechanical and hydrothermal stabilities of aged periodic mesoporous organosilicas. J Phys Chem B 107:12628–12634. https://doi.org/10.1021/jp035189q
Li N, Chen J, Shi YP (2017) Magnetic polyethyleneimine functionalized reduced graphene oxide as a novel magnetic solid-phase extraction adsorbent for the determination of polar acidic herbicides in rice. Anal Chim Acta 949:23–34. https://doi.org/10.1016/j.aca.2016.11.016
Acknowledgements
Financial support from Tarbiat Modares University is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 1715 kb)
Rights and permissions
About this article
Cite this article
Mousavi, K.Z., Yamini, Y., Karimi, B. et al. Imidazolium-based mesoporous organosilicas with bridging organic groups for microextraction by packed sorbent of phenoxy acid herbicides, polycyclic aromatic hydrocarbons and chlorophenols. Microchim Acta 186, 239 (2019). https://doi.org/10.1007/s00604-019-3355-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3355-3