2.1. Weight-Loss Measurements and SEM/EDX Investigations
The variation of (a) the weight-loss (Δm, mg/in
2) and (b) the corrosion rate (
RCorr, mpy)
vs. time for the cast iron coupons in 300 cm
3 of aerated stagnant solutions of AGS are shown in
Figure 1. The values of Δm and
RCorr over the exposure time were calculated as reported in our previous work as following [
11]:
where, m
1 and m
2 are the weighs of the cast iron coupon per mg before and after its immersion in the test solution,
A is the area of the cast iron coupon in inch
2,
D is the density of cast iron (
D = 7.563 g/cm
3), and
t is the exposure time (h).
Figure 1.
Change of (a) the dissolution rate (Δm, mg/in2) and (b) the corrosion rate (RCorr, mpy) with time for the cast iron coupons in the Arabian Gulf seawater (AGS) solutions.
Figure 1.
Change of (a) the dissolution rate (Δm, mg/in2) and (b) the corrosion rate (RCorr, mpy) with time for the cast iron coupons in the Arabian Gulf seawater (AGS) solutions.
One can see from
Figure 1a that the values of Δm increased with time due to the aggressiveness attack of the corrosive ions present in the seawater toward the cast iron surface. It is well known that the cathodic reaction for metals and alloys in near neutral solutions is the oxygen reduction according to the following equation:
On the other hand, the anodic reaction of iron in aerated neutral solutions is the dissolution of metallic iron (Fe°) to ferrous cations (Fe
2+) as follows,
The dissolution of iron gets activated due to the consumption of the produced electrons via the cathodic reaction at the anodic one, which could lead to the increased weight-loss with time,
Figure 1a. Similar to the increase of Δm with time, the values of K
Corr also increased with increasing the immersion time, particularly at short periods, as can been seen from
Figure 1b. This increment slightly decreased at longer immersion time due to the formation of thick corrosion products that decreases the attack of the corrosive species present in the seawater and thus decreases the corrosion rate.
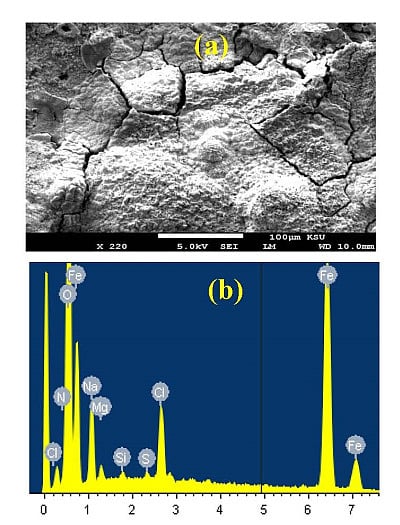
In order to understand the morphology and the composition of the corrosion products formed on the cast iron coupon after its immersion in the seawater for 10 days, SEM/EDX investigations for the surface of the coupon were carried out.
Figure 2a shows the SEM image obtained for the cast iron surface at different magnifications after 10 days’ immersion in the aerated stagnant AGS solution, and
Figure 2b represents the EDX profile analysis for the area of the surface shown in
Figure 2a. It is clearly seen from the SEM image that the surface of the cast iron is fully covered with thick layers of corrosion products due to the immersion of the coupon in AGS for long time, 10 days. The atomic percentages of the components found on the surface were 56.87% O, 32.57% Fe, 9.64% Na, 3.50% Cl, 1.04% Mg, 0.29% Si, and 0.27% S. The very high content of oxygen and iron indicates that the layers formed on the cast iron surface after 10 days immersion in AGS were most probably iron oxide films. This can be explained on the light of the overall reaction that occurs on the iron surface as follows,
This ferrous hydroxide formed layer reacts, further in the presence of excess oxygen in the solution, to building up the final corrosion product Fe
3O
4 (magnetite) according to the following reaction [
12];
Figure 2.
(a) SEM micrograph obtained for the cast iron coupons after its immersion in AGS for 10 days; and (b) the EDX profile analysis obtained for the surface shown in the SEM image.
Figure 2.
(a) SEM micrograph obtained for the cast iron coupons after its immersion in AGS for 10 days; and (b) the EDX profile analysis obtained for the surface shown in the SEM image.
According to Mohebbi and Li in a similar study [
13], both Fe
3+ and Fe
2+ ion species existed in the corrosion product, which indicates that the electrons generated by oxidation of iron (cathodic reaction) can be readily consumed by the oxygen present in the electrolyte solution through the porous conductive corrosion layer. The presence of Cl ions gives indications on the increase of weight-loss and corrosion rate of cast iron with time due to possible reactions of Cl
− with the inner surface of iron [
13]. This is due to the dissolution of iron as shown in Equation (4), which would lead to the formation of FeCl
2 and FeC1
3 in the solution. The concentration of FeCl
2 and FeC1
3 at this condition is at saturation and would precipitate to form a porous mixed film(s) of FeCl
2 and FeC1
3 on the iron electrode surface [
14],
In addition to the iron oxides, the presence of Mg, Na and Si indicates that their oxides might have also formed on the surface.
2.2. Cyclic Potentiodynamic Polarization (CPP) Measurements
Cyclic potentiodynamic polarization testing were conducted to measure the corrosion parameters such as cathodic (
βc) and anodic (
βa) Tafel slopes, corrosion potential (
ECorr), pitting potential (
EPit), protection potential (
EProt), corrosion current (
jCorr), polarization resistance (
RP) and corrosion rate (
RCorr).
Figure 3 shows the CPP curves obtained for the cast iron electrode after its immersion in AGS solutions for (a) 1 h and (b) 24 h, respectively. The corrosion parameters obtained from the CPP curves shown in
Figure 3 are list in
Table 1. The values of
βc and
βa were determined after at least 50 mV away from E
Corr and at least one decade of current densities (
jCorr). The values of
ECorr and
jCorr were obtained from the intersection of the extrapolation of anodic and cathodic Tafel lines located next to the linearized current regions. The values of polarization resistance,
Rp, and corrosion rate,
RCorr, for the cast iron were calculated according to the following equations [
12,
15] corrosion as follows:
Where, k is a constant that defines the units for the corrosion rate (=3272 mm amp
1cm
−1 y
−1); E
W the equivalent weight in grams/equivalent of cast iron alloy (E
W = 27.9 grams/equivalent); d the density in gcm
−3 (=7.563); and A the area of the exposed surface of the electrode in cm
2.
It is clearly seen from
Figure 3 that the anodic branch for the cast iron shows a passive region whether the measurement was taken after 1 h (
Figure 3a) or after 24 h (
Figure 3b). This passive region was formed due to the formation of an oxide film as depicted by Equations (5) and (6). Where, the formed ferrous hydroxide reacted with more oxygen to form the top layer of magnetite corrosion product, Fe
3O
4. The current then increased rapidly in the anodic side due to the breakdown of the formed oxide film and the occurrence of pitting corrosion. This was indicated by the higher current values in the backward direction of the scanned potential and the appearance of a hysteresis loop, the area of which decreased by increasing immersion time from 1h to 24 h. It is well known that AGS contains corrosive species such as chloride ions, which attacks the weak and flowed areas of the oxide film formed on the iron surface in the passive region causing its breakdown and corrosion via pitting by chloride ions attack as depicted in Equations (7) and (8).
Figure 3.
Cyclic potentiodynamic polarization curves obtained for the cast iron electrode after its immersion in AGS solutions for (a) 1 h and (b) 24 h, respectively.
Figure 3.
Cyclic potentiodynamic polarization curves obtained for the cast iron electrode after its immersion in AGS solutions for (a) 1 h and (b) 24 h, respectively.
Table 1.
Polarization parameters obtained for the cast iron electrode after its immersion for 1 h and 24 h in the Arabian Gulf seawater (AGS).
Table 1.
Polarization parameters obtained for the cast iron electrode after its immersion for 1 h and 24 h in the Arabian Gulf seawater (AGS).
| Immersion time | Polarization Parameters |
|---|
βc/
mVdec−1 | ECorr/
mV | βa/
mV/dec−1 | jCorr/
µA cm−2 | EPit/
mV | EProt/
mV | Rp/
Ω cm2 | RCorr/
mmpy |
|---|
| AGS (1 h) | 95 | −860 | 230 | 20 | −630 | −665 | 1471 | 0.2414 |
| AGS (24 h) | 105 | −825 | 210 | 11 | −620 | −680 | 2767 | 0.1328 |
The parameters shown in
Table 1 show that the increase of immersion time from 1h to 24 h shifted
ECorr and
EPit to the less negative values, while
EProt was shifted to the more negative values. This indicates that both general and pitting corrosion decreased with increasing the time of immersion for the cast iron in AGS.
Table 1 also shows that the increase of time before measurements decreased the values of j
Corr and
RCorr as well as increased the polarization resistance (
Rp).
2.3. Open-Circuit Potential (OCP) and Electrochemical Impedance Spectroscopy (EIS) Measurements
Figure 4 shows the change of the OCP with time for the cast iron electrode in the AGS solution. It is seen from
Figure 4 that the initial potential of iron rapidly increased towards the more negative values in the first few minutes due to the dissolution of a preformed air oxide film. The potential then slightly shifted in the more negative direction with the appearance of some fluctuations by increasing time for the first 12 h. This more negative shift might have resulted from the dissolution of iron by the corrosive ions attack such as chlorides. Finally, the potential very slightly decreases again towards the less negative direction till the end of the experiment as a result of the oxide and/or corrosion product layers on the surface.
Figure 4.
Change of the open-circuit potential with time for the cast iron electrode in the AGS solution.
Figure 4.
Change of the open-circuit potential with time for the cast iron electrode in the AGS solution.
EIS method is a powerful technique that has been used in studying corrosion and corrosion inhibition of metals and alloys in various corrosive media [
14,
15,
16,
17,
18,
19,
20,
21]. Typical Nyquist obtained for the cast iron electrode after (1) 1 h and (2) 24 h immersion in the AGS solution, respectively, are shown in
Figure 5. The spectra represented in
Figure 5 were analyzed by best fitting to the equivalent circuit model depicted in
Figure 6. The EIS parameters obtained by fitting this circuit are listed in
Table 2 and can be defined according to the usual convention as follows; R
S represents the solution resistance, Q is the constant phase elements (CPEs), Cdl is the double layer capacitance, Rp
1 is the polarization resistance for the solution/cast iron interface and may be defined as the charge transfer resistance of the cathodic reduction reaction of the cast iron, and Rp
2 is another polarization resistance for the corrosion product/cast iron interface [
22].
Figure 5.
Typical Nyquist obtained for the cast iron electrode after (1) 1 h and (2) 24 h immersion in the AGS solution, respectively.
Figure 5.
Typical Nyquist obtained for the cast iron electrode after (1) 1 h and (2) 24 h immersion in the AGS solution, respectively.
Figure 6.
The equivalent circuit model used to the fit the EIS data shown in
Figure 5; the symbols of the equivalent circuit are defined in the text and the values are listed in
Table 2.
Figure 6.
The equivalent circuit model used to the fit the EIS data shown in
Figure 5; the symbols of the equivalent circuit are defined in the text and the values are listed in
Table 2.
Table 2.
Parameters obtained by fitting the EIS data shown in
Figure 5 with the equivalent circuit shown in
Figure 6 for the electrode after its immersion for 1 h and 24 h in AGS.
Table 2.
Parameters obtained by fitting the EIS data shown in Figure 5 with the equivalent circuit shown in Figure 6 for the electrode after its immersion for 1 h and 24 h in AGS.
| Immersion Time | Kinetic EIS Parameters |
|---|
RS/
Ω cm2 | Q | RP1/
Ω cm2 | Cdl/
F cm−2 | RP2/
Ω cm2 |
|---|
| YQ/F cm−2 | n |
|---|
| AGS (1 h) | 5.814 | 0.000948 | 0.72 | 0.3977 | 5.232 × 10−5 | 1047 |
| AGS (24 h) | 6.127 | 0.000539 | 0.72 | 40.36 | 2.374 × 10−5 | 1571 |
It is seen from
Figure 5 that the cast iron showed only one semicircle whether the immersion time for the cast iron before measurement was 1 h or 24 h. The diameter of the obtained semicircle got wider by increasing the immersion time to 24 h, which indicates that the resistance against corrosion increased by increasing the exposure period of time from 1h to 24 h. The values of
RS,
Rp
1 and
Rp
2 that are listed in
Table 2 recorded higher values for the cast iron immersed in AGS for 24 h compared to those obtained after only 1 h. This is due to the increase of the corrosion resistance for the surface of the cast iron with increasing time. The constant phase elements (
Q, CPEs) with its n values exactly 0.72 for the cast iron after 1 h and 24 h immersion in the AGS electrolyte represent double layer capacitors with some pores that allow the dissolution of iron [
14,
15,
16,
17], which agrees with the work of Mohebbi and Li [
13]. Where and depending on the value of
n, a CPE can represent resistance (
Z(CPE) =
R,
n = 0), capacitance (
Z(CPE) = Cdl,
n = 1) or Warburg impedance for (
n = 0.5). Therefore, the CPE for iron and steel is substituted for the capacitor to fit the semicircle more accurately. According to Zhang
et al., the admittance and the impedance of a CPE at this condition can be defined by the following equations, respectively [
16].
where,
Y0 is the modulus; ω is the angular frequency; and n is the phase. The decrease of the CPEs and
Cdl values byincreasing the immersion period to 24 h reveals also that elongating time lowers the corrosion of the cast iron [
16,
23].
Figure 7 shows the typical Bode (
Figure 7a) impedance of the interface, |
Z|, and (
Figure 7b) phase angle plots obtained for the cast iron electrode after (1) 1 h and (2) 24 h immersion in the AGS solution, respectively. It is obvious that the increase of immersion time to 24 h before measurements increased the impedance |
Z| values (
Figure 7a). It has been reported by Mansfeld
et al. [
24] that the surface is more protected when higher |
Z| values are shown, particularly at the low frequency region. The increase of immersion time also increased the maximum degree of the phase angle as can be seen from
Figure 7b. This confirms that the better corrosion resistance of the cast iron with increasing time. The EIS Nyquist and Bode plots were consistent with each other and both are in good agreement with the data obtained by weight-loss and cyclic polarization measurements.
Figure 7.
Typical Bode (a) impedance of the interface, |Z|; and (b) phase angle plots obtained for the cast iron electrode after (1) 1 h and (2) 24 h immersion in the AGS solution, respectively.
Figure 7.
Typical Bode (a) impedance of the interface, |Z|; and (b) phase angle plots obtained for the cast iron electrode after (1) 1 h and (2) 24 h immersion in the AGS solution, respectively.