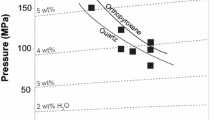
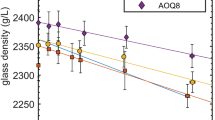
Abstract
Fluid/melt distribution coefficients for F have been determined in experiments conducted with peraluminous topaz rhyolite melts and fluids consisting of H2O and H2O+CO2 at pressures of 0.5 to 5 kbar, temperatures of 775°–1000°C, and concentrations of F in the melt ranging from 0.5 to 6.9 wt%. The major element, F, and Cl concentrations of the starting material and run product glasses were determined by electron microprobe, and the concentration of F in the fluid was calculated by mass balance. The H2O concentrations of some run product glasses were determined by ion microprobe (SIMS). The solubility of melt in the fluid phase increases with increasing F in the system; the solubility of H2O in the melt is independent of the F concentration of the system with up to 6.3 wt% F in the melt. No evidence of immiscible silica- and fluoriderich liquids was detected in the hydrous but water-undersaturated starting material glasses (≦8.5 wt% F in melt) or in the water-saturated run product glasses. F concentrates in topaz rhyolite melts relative to coexisting fluids at most conditions studied; however, DF (wt% F in fluid/wt% F in melt) increases strongly with increasing F in the system. Maximum values of DF in this study are significantly larger than those previously reported in the literature. Linear extrapolation of the data suggests that DF is greater than one for water-saturated, peraluminous granitic melts containing ≧8 wt% F at 800° C and 2 kbar. DF increases as temperature and as (H2O/H2O+CO2) of the fluid increase. For topaz rhyolite melts containing ≦1 wt% F and with H2O-rich fluids, DF is independent of changes in pressure from 2 to 5 kbar at 800° C; for melts containing ≦1 wt% F and in equilibrium with CO2-bearing fluids the concentrations of F in fluid increases with increasing pressure. F-and lithophile element-enriched granites may evolve to compositions containing extreme concentrations of F during the final stages of crystallization. If F in the melt exceeds 8 wt%, DF is greater than one and the associated magmatic-hydrothermal fluid contains >4 molal F. Such F-enriched fluids may be important in the mass transport of ore constituents, i.e., F, Mo, W, Sn, Li, Be, Rb, Cs, U, Th, Nb, Ta, and B, from the magma.
Similar content being viewed by others
References
Anfiligov VN, Glyuk DS, Trufanova LG (1973) Phase relations in the interaction between granite and sodium fluoride at water vapor pressure of 1,000 kg/cm2. Geokhim 1:44–48
Bailey JC (1977) Fluorine in granitic rocks and melts: a review. Chem Geol 19:1–42
Barton MD (1982) The thermodynamic properties of topaz solid solutions and some petrologic applications. Am Mineral 67:956–974
Barton MD (1987) Lithophile-element mineralization associated with Late Cretaceous two-mica granites in the Great Basin. Geol 15:337–340
Bowden P, Whitley JE (1974) Rare-earth patterns in peralkaline and associated granites. Lithos 7:15–21
Brimhall GH, Crerar DA (1978) Ore fluids: Magmatic to supergene: In: ISE Carmichael, HP Eugster (eds) Thermodynamic modeling of geological materials: minerals, fluids and melts. Mineral Soc Am Rev Mineral 17:235–322
Burnham CW (1967) Hydrothermal fluids at the magmatic stage: In: HL Barnes (ed) Geochemistry of hydrothermal ore deposits. Wiley, New York, pp 34–76
Burnham CW (1981) Nature of multicomponent aluminosilicate melts. In: DT Rickard, EF Wickmann (eds) Chemistry and geochemistry of solutions at high temperatures and pressures. Pergamon Press 13&14, Oxford New York, pp 197–229
Burt DM, Sheridan MF (1986) Mineral deposits related to topaz rhyolites in the southwest. Ariz Geol Soc Dig XVI:170–178
Burt DM, Bikun JV, Christiansen EH (1982) Topaz rhyolites-distribution, origin, and significance for exploration. Econ Geol 77:1818–1836
Candela PA, Holland HD (1984) The partitioning of copper and molybdenum between silicate melts and aqueous fluids. Geochim Cosmochim Acta 48:373–380
Carten RB, Geraghty EP, Walker BM, Shannon JR (1988) Cyclic development of igneous features and their relationship to hightemperature hydrothermal features in the Henderson Porphyry molybdenum deposit, Colorado. Econ Geol 83:266–296
Christiansen EH, Sheridan MF, Burt DM (1986) The geology and geochemistry of Cenozoic topaz rhyolites from the western United States. Geol Soc Am Spec Pap 205, 82 p
Clark SP, Jr (1966) Handbook of physical constants. Geol Soc Am Mem 97:432–435
Coats RR, Goss WD, Rader LF (1963) Distribution of fluorine in unaltered volcanic rocks of the western conterminous United States. Econ Geol 58:941–951
Dayvault RD, Rush SM, Ludlam JR (1983) Evaluation of uranium potential in a topaz-bearing rhyolite, China Hat Dome, Southeastern Idaho. Bend Fd Eng Corp Rpt GJBX-1(84), p 26
Dingwell DB (1984) Investigations of the role of fluorine in silicate melts: implications for igneous petrogenesis. Unpubl PhD thesis, Univ Alberta, Edmonton, p 149
Dingwell DB (1988) Effect of fluorine on the viscosity of diopside melt. abs EOS 69:1468
Dingwell DB, Scarfe CM, Cronin DJ (1985) The effect of fluorine on viscosities in the system Na2O−Al2O3−SiO3: implications for phonolytes, trachytes, and rhyolites. Am Mineral 70:80–87
Flynn RT, Burnham CW (1978) An experimental determination of rare earth partition coefficients between a chloride containing vapor phase and silicate melts. Geochim Cosmochim Acta 42:658–701
Foley SF, Taylor WR, Green DH (1986) The effect of fluorine on phase relationships in the system KAlSiO4−Mg2SiO4−SiO2 at 28 kbar and the solution mechanism of fluorine in silicate melts. Contrib Mineral Petrol 93:46–55
Glyuk DS, Anfiligov VN (1983) Phase equilibria in the system granite-H2O−HF at a pressure of 1000 kg/cm2. Geokhim 3:321–325
Gunow AJ (1978) The geochemistry of fluorine-rich micas at the Henderson molybdenite deposit: Unpubl MSc thesis, Univ Colorado, Boulder, p 113
Gunow AJ, Ludington S, Munoz JL (1980) Fluorine in micas from the Henderson molybdenite deposit, Colorado. Econ Geol 75:1127–1137
Hards NJ (1978) Distribution of elements between the fluid phase and silicate melt phase of granites and nepheline syenites: In: CMB Henderson (ed) Progress in experimental petrology. NERC Prog Exp Pet 3:88–90
Holland HD (1972) Granites, solutions and base metal deposits. Econ Geol 67:281–301
Holloway JR (1978) Igneous fluids: In: ISE Carmichael, HP Eugster (eds) Thermodynamic modeling of geological materials: minerals, fluids and melts. Mineral Soc Am Rev Mineral 17:211–234
Holloway JR, Ford CE (1975) Fluid absent melting of the fluorhydroxy amphibole pargasite to 35 kbar. Earth Planet Sci Lett 25:44–48
Huebner JS (1971) Buffering techniques for hydrostatic systems at elevated pressures. In: GC Ulmer (ed) Research techniques for high pressure and high temperature. Springer, New York Berlin Heidelberg, pp 123–176
Koster van Groos AFK, Wyllie PJ (1967) Melting relationships in the system, NaAlSi3O8−NaF−H2O to 4 kb pressure. J Geol 76:50–70
Kovalenko NI (1977) The reactions between granite and aqueous hydrofluoric acid in relation to the origin of fluorine-bearing granites. Geochim 4:503–515
Lamba VJS, Agarkar PS (1988) The tin potential of Precambrian rare-metal bearing pegmatites of Bastar District, MP, India. Mineral Dep 23:218–221
Lindsey DA (1982) Tertiary volcanic rocks and uranium in the Thomas Range and northern Drum Mountains, Juab County Utah. US Geol Surv Prof Pap 1221:71
London D (1987) Internal differentiation of rare-element pegmatites: effects of boron, phosphorus, and fluorine. Geochim Cosmochim Acta 51:403–420
Manning DAC (1981) The effect of fluorine on liquidus phase relationships in the system Qz−Ab−Or with excess water at 1 kbar. Contrib Mineral Petrol 76:257–262
Manning DAC, Henderson P (1984) The behaviour of tungsten in granitic melt-vapour systems: Contrib Mineral Petrol 86:286–293
Morey GW, Chen WT (1956) Pressure-temperature curves in some systems containing water and a salt. Jour Amer Chem Soc 78:4249–4252
Munoz JL, Ludington SD (1974) Fluoride-hydroxyl exchange in biotite. Am J Sci 274:396–413
Mutschler FE, Wright EG, Ludington S, Abbott JT (1981) Granite molydenite systems. Econ Geol 76:874–897
Nash WP, Congdon R (1987) Accessory minerals in high fluorine rhyolite: Composition and partition coefficients. abs EOS 68:1513
Nekvasil H (1986) A theoretical thermodynamic investigation of the system Ab−Or−An−Qz(H2O) and implications for melt speciation. Unpubl PhD thesis, The Penn State Univ, University Park, p 268
Noble DC, Smith VC, Peck LC (1967) Loss of halogens from crystallized and glassy silicic volcanic rocks. Geochimi Cosmochim Acta 31:215–223
Payette C, Martin RF (1989) Chemical features of felsic magmas erupted in the Thomas Range, Utah, as revealed by melt inclusions. Geol Soc Am Spec Pap (in press)
Rosholt JN, Prijana, Noble DC (1971) Mobility of uranium and thorium in glassy and crystallized silicic volcanic rocks. Econ Geol 66:1061–1069
Rubin JN, Price JG, Henry CD, Koppenaal DW (1987) Cryolitebearing and rare metal-enriched rhyolite, Sierra Blanca Peaks, Hudspeth County, Texas. Am Mineral 72:1122–1130
Sharp JE (1978) A molybdenum mineralized breccia pipe complex, Redwell Basin, Colorado. Econ Geol 73:369–382
Sharp JE (1979) Cave Peak, a molybdenum-mineralized breccia pipe complex in Culberson County, Texas. Econ Geol 74:517–534
Shaw H (1963) Hydrogen-water vapor mixtures: control of hydrothermal atmospheres by hydrogen osmosis. Science 139:1220–1222
Smith FG (1948) Transport and deposition of the non-sulphide vein minerals. III Phase relations at the pegmatitic stage. Econ Geol XLII:525–546
Sorapure R, Hamilton DL (1984) The solubility of water in melts of albite composition with varying additions of fluorine. In: CMB Henderson (ed) Progress in experimental petrology. NERC Prog Exp Pet 6:28–29
Swanson SE, Bond JF, Newberry RJ (1988) Petrogenesis of the Ear Mountain tin granite, Seward Peninsula, Alaska. Econ Geol 83:46–61
Wallace SR, MacKenzie WB, Blair RG, Muncaster NK (1978) Geology of the Urad and Henderson molybdenite deposits, Clear Creek County, Colorado, with a section on a comparison of these deposits with those at Climax, Colorado. Econ Geol 73:325–368
Webster JD, Duffield, WA (1990) Pre-eruptive concentrations of volatiles and lithophile trace and ore elements in Taylor Creek Rhyolite, New Mexico: Analysis of glass inclusions in quartz phenocrysts. Am Mineral (in press)
Webster JD, Holloway JR (1987) Partitioning of halogens between topaz rhyolite melt and aqueous and aqueous-carbonic fluids. Geol Soc Am Abst Progr 19:884
Webster JD, Holloway JR (1988) Experimental constraints on the partitioning of Cl between topaz rhyolite melt and H2O and H2O+CO2 fluids: new implications for granitic differentiation and ore deposition. Geochim Cosmochim Acta 52:2091–2105
Webster JD, Holloway JR (1989) Partitioning of F and Cl between magmatic-hydrothermal fluids and highly evolved granitic magmas: In: HJ Stein, JL Hannah (eds) Ore-bearing granite systems; petrogenesis and mineralizing processes. Geol Soc Am Spec Pap 246 (in press)
Webster JD, Holloway JR, Hervig RL (1987) Phase equilibria of a Be, U and F-enriched vitrophyre from Spor Mountain, Utah. Geochim Cosmochim Acta 51:389–402
Webster JD, Gillen G, Simons DS (1988) Lithophile element transport in Cl- and F-bearing magmatic-hydrothermal fluids derived from highly differentiated granitic magmas: chlorophile versus fluorophile elements. abs EOS 69:515
Westra G, Keith SB (1981) Classification and genesis of stockwork molybdenum deposits. Econ Geol 76:844–873
White WH, Bookstrom AA, Kamilli RJ, Ganster MW, Smith RP, Ranta DE, Steininger RC (1981) Character and origin of Climax-type molybdenum deposits. Econ Geol 75:270–316
Witt WL (1988) Evolution of high-temperature hydrothermal fluids associated with greisenization and feldspathic alteration of a tin-mineralized granite, northeast Queensland. Econ Geol 83:310–334
Zielinski RA, Lipman PW, Millard HT (1977) Minor element abundances in obsidian, perlite, and felsite of cale-alkalic rhyolites. Am Mineral 62:426–437
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Webster, J.D. Partitioning of F between H2O and CO2 fluids and topaz rhyolite melt. Contr. Mineral. and Petrol. 104, 424–438 (1990). https://doi.org/10.1007/BF01575620
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01575620