Summary
The pharmacokinetics and haemodynamic effects of orally administered spirapril, a novel angiotensinconverting enzyme (ACE) inhibitor, have been investigated in patients with liver cirrhosis (n=10), in patients with chronic, non-cirrhotic liver disease (n=8) and in a control group of healthy subjects (n=16).
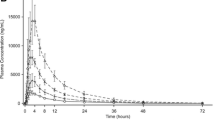
The absorption and elimination of spirapril did not differ between patients with liver disease and control subjects. In contrast, the bioavailability of spiraprilat, the metabolite responsible for the pharmacological action of spirapril, was significantly reduced in patients (AUC 820 μg·h·l−1, 923 μg·h·l−1 and 1300 μg·h·l−1 in patients with cirrhosis, patients with non-cirrhotic liver disease and in healthy subjects, respectively.
Compared to healthy subjects, cirrhotic patients had a reduced rate constant of spiraprilat formation (1.10 h−1 in patients vs. 2.00 h−1 in control subjects) while the elimination half-life of spiraprilat was not different. The effect of spirapril on diastolic blood pressure was decreased in patients with chronic liver disease as compared to the controls.
Thus, the pharmacokinetics of spirapril was unchanged in patients with different types of liver disease, including cirrhosis. However, the bioavailability of spiraprilat and hypotensive effect of spirapril were reduced in patients.
Similar content being viewed by others
References
Beyer KH, Peuler JD (1982) Hypertension: perspectives. Pharmacol Rev 34: 287–313
Johnston CI, Arnolda L, Hiwatari M (1984) Angiotensin-converting enzyme inhibitors in the treatment of hypertension. Drugs 27: 271–277
The Consensus Trial Study Group (1987) Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (Consensus). N Engl J Med 316: 1429–1435
The SOLVD Investigators (1991) Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 325: 293–302
Cohn JN, Johnson G, Ziesche S (1991) A comparison of enalapril with hydralazine-isosorbide dinatrate in the treatment of chronic congestive heart failure. N Engl J Med 325: 303–310
Todd PA, Heel RC (1986) Enalapril. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in hypertension and congestive heart failure. Drugs 31: 198–248
Breckenridge A (1988) Angiotensin converting enzyme inhibitors. BMJ 296: 618–620
Monopoli A, Forlani A, Milani S, Ongini E (1988) Antihypertensive effect of spirapril and felodipine during repeated administration to spontaneously hypertensive rats. Pharmacol Res Commun 20: 37–47
Sybertz EJ, Watkins RW, Ahn HS, Baum T, La-Rocca P, Patrick J, Leitz F (1987) Pharmacologic, metabolic and toxicologic profile of spirapril (SCH 33844), a new angiotensin converting inhibitor. J Cardiovasc Pharmacol 10: S105-S108
Sybertz EJ, Baum T, Ahn HS, Desiderio DM, Pula KK, Tedesco R, Washington P, Sabin C, Smith E, Becker F (1987) Angiotensin converting enzyme inhibitory activity of SCH 33844 (spirapril) in rats, dogs and monkeys. Arch Int Pharmacodyn Ther 286: 216–229
Van den Meiracker AH, Man in't Veld AJ, Ritsema van Eck HJ, Schalekamp MA (1989) Comparison of the acute and chronic antihypertensive effect of two once-daily doses of spirapril by invasive twenty-four-hour ambulatory blood pressure monitoring. J Hypertens 7: S302-S303
Van den Broek SA, van Bruggen A, de Graeff PA, Hillege H, van Gilst WH, Wesseling H, Lie KI (1991) The acute hemodynamic, hormonal, and pharmacokinetic properties of oral spirapril in patients with moderate to severe heart failure. J Cardiovasc Pharmacol 18: 614–621
Hof RP, Hof-Miyashita A, Evenou JP (1987) Hemodynamic effects of a new angiotensin converting enzyme inhibitor, spirapril, in sodium loaded and sodium depleted rabbits. J Cardiovasc Pharmacol 10: 599–606
Smith EM, Swiss GF, Neustadt BR, McNamara P, Gold EH, Sybertz EJ, Baum T (1989) Angiotensin converting enzyme inhibitors: spirapril and related compounds. J Med Chem 32: 1600–1606
Hossein-Nia M, Surve A, Weglein R, Gerbeau C, Holt D (1992) Radioimmunoassays for spirapril and its active metabolite spiraprilat: performance and application. Ther Drug Monit 14: 234–242
Tygstrup N (1963) Determination of hepatic galactose elimination capacity after a single intravenous injection in man. Acta Physiol Scand 58: 162–172
Wahlländer A, Renner E, Preisig R (1985) Fasting plasma caffeine concentration. A guid to the severity of chronic liver disease. Scand J Gastroenterol 20: 1133–1141
Bircher J, Küpfer A, Gikalov I, Preisig R (1976) Aminopyrine demethylation measured by breath analysis in cirrhosis. Clin Pharmacol Ther 20: 484–492
Armitage P, Berry G (1987) Statistical methods in medical research. Blackwell, Oxford London Edinburgh
Blaschke TF, Rubin PC (1979) Hepatic first-pass metabolism in liver disease. Clin Pharmacokinet 4: 423–432
Pond SM, Tozer NT (1984) First-pass elimination. Basic concepts and clinical consequences. Clin Pharmacokinet 9: 1–25
Wilkinson GR, Shand DG (1975) A physiological approach to hepatic drug clearance. Clin Pharmacol Ther 18: 377–390
Lauterburg BH, Bircher J (1976) Exspiratory measurement of maximal aminopyrine demethylation in vivo: effect of phenobarbital, partial hepatectomy, portacaval shunt and bile duct ligation in the rat. J Pharmacol Exp Ther 196: 501–509
Reichen J, Egger B, Ohara N, Zeltner TB, Zysset T, Zimmermann A (1988) Determinants of hepatic function in liver cirrhosis in the rat. Multivariate analysis. J Clin Invest 82. 2069–2076
Arndt R, Heymann E, Junge W, Krisch K, Hollandt H (1973) Purification and molecular properties of an unspecific carboxylesterase (E1) from rat-liver microsomes. Eur J Biochem 36: 120–128
Pang KS, Barker F, Cherry WF, Goresky CA (1991) Esterases for enalapril hydrolysis are concentrated in the perihepatic venous region of the rat liver. J Pharmacol Exp Ther 257: 294–301
Ohkubo H, Okuda K, Iida S, Ohnishi K, Ikawa S, Makino I (1984) Role of portal and splenic vein shunts and impaired hepatic extraction in the elevated serum bile acids in liver cirrhosis. Gastroenterology 86: 514–520
Paumgartner G (1975) The handling of indocyanine green by the liver. Schweiz Med Wochenschr 105: S1-S30
Ohnishi A, Tsuboi Y, Ishizaki T, Kubota K, Ohno T, Yoshida H, Kanezaki A, Tanaka T (1989) Kinetics and dynamics of enalapril in patients with liver cirrhosis. Clin Pharmacol Ther 45: 657–665
Carr RD, Cooper AE, Hutchinson R, Mann J, O'Conner SE, Robinson DH, Wells E (1990) Preferential biliary elimination of FPL 63547, a novel inhibitor of angiotensin-converting enzyme, in the rat. Br J Pharmacol 100: 90–94
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Krähenbühl, S., Grass, P., Surve, A. et al. Pharmacokinetics and haemodynamic effects of a single oral dose of the novel ACE inhibitor spirapril in patients with chronic liver disease. Eur J Clin Pharmacol 45, 247–253 (1993). https://doi.org/10.1007/BF00315391
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00315391