Abstract
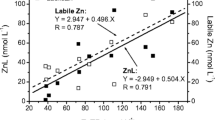
Concentrations of Ca, Cd, Cu, Fe, and Pb and binding capacities for Cd, Cu, and Pb were determined for water samples collected from 12 lakes in southwestern Maine using atomic absorption and ion-selective electrodes, respectively. Surface waters in this area are soft and characterized by low pH. Some lakes were highly colored with refractory organics, whereas others had very low organic carbon concentrations. Both Cu and Pb concentrations were positively correlated with organic carbon content. Copper and Pb binding capacities were significantly correlated with organic carbon content, whereas Cd binding capacity was positively correlated with pH, but not with organic carbon. Surface water binding capacity for Pb was greater than that for Cu or Cd. More than 99% of the Ca from all waters studied was removed onto cationic exchange resins. Less than 1% of the total Cu existed as cationic species in highly humic waters, whereas as much as 65% existed as cationic species in less huic waters. Conversely, more than 99% of the Cd present in humic waters existed as cationic species, whereas as little as 50% existed as cationic species in less humic, more alkaline waters. These correlative studies indicate that binding by organic carbon is important for both Cu and Pb in this area and must be considered in models of trace metal speciation. However, Cd binding in the same waters is not correlated with organic carbon, which does not need to be considered in speciation models for Cd.
Similar content being viewed by others
References
Akiyama, T., 1973, Interactions of ferrous ions and organic matter in water environments: Geochim. Jour., v. 7, pp. 167–177.
Allen, H. L., 1976, Dissolved organic matter in lakewater: characteristics of molecular weight size fractions and ecological implications: Oikos, v. 27, pp. 64–70.
Andrew, R. W., K. E. Biesinger, and G. E. Glass, 1977, Effects of inorganic complexing on the toxicity of copper toDaphnia magna: Water Res., v. 11, pp. 309–315.
Anon., 1974, Lead electrode model 94-82 instruction manual: Orion Research, Inc., 22 p.
Anon., 1975a, Cadmium electrode model 94-38 instruction manual: Orion Research, Inc., 28 p.
Anon., 1975b, Cupric electrode model 94-29 instruction manual: Orion Research, Inc., 28 p.
American Public Health Association, 1976, Standard methods for the examination of water and waste water: 14th ed., 1193 p.
Barr, A. J., J. H. Goodnight, J. P. Sall, and J. T. Helwig, 1976, A user guide to SAS-76: Raleigh, North Carolina, SAS Institute Incorporated, 329 p.
Bauman, E. W., 1976, Nephelometric determination of microgram quantities of sulfate with 2-aminoperimidine: Savannah River Laboratory ERDA, DP-1437, 17 p.
Beck, K. C., J. H. Reuter, and E. M. Perdue, 1974, Organic and inorganic geochemistry of some coastal plain rivers of the southeastern United States: Geochim. Cosmochim. Acta, v. 38, pp. 341–364.
Benes, P., E. T. Gjessing, and E. Steinnes, 1976, Interactions between humus and trace elements in freshwater: Water Res., v. 10, pp. 711–716.
Briese, L. A., and J. P. Giesy, 1975, Determination of lead and cadmium associated with naturally occurring organics extracted from surface waters, using flameless atomic absorption: Atomic Absor. Newslett v. 14, pp. 133–136.
Brown, V. M., T. L. Shaw, and D. G. Shurben, 1974, Aspects of water quality and the toxicity of copper to rainbow trout: Water Res., v. 8 pp. 797–803.
Clubb, R. W., A. R. Gaufin, and J. L. Lords, 1975, Acute cadmium toxicity studies upon nine species of aquatic insects: Environ. Res., v. 9, pp. 332–341.
Davies, A. G., 1970, Iron, chelation and the growth of marine phytoplankton, I: Growth kinetics and chlorophyll production in cultures on the euryhaline flagellateDunaliella tertiolecta under iron limiting conditions: Mar. Biol. Assoc. Jour., U.K., v. 40, pp. 65–86.
Durum, W. H., 1974, Occurrence of some trace metals in surface and groundwater: Univ. Illinois, Urbana-Champaign, Ill., Water Quality Conference, Proc., v. 16, pp. 17–25.
Elder, J. F., 1975, Complexation side reactions involving trace metals in natural water systems: Limnol. Oceanogr., v. 20, pp. 96–102.
Ellis, B. G., and B. D. Knezek, 1972, Adsorption reactions of micronutments in soils,in Mortvedt, J., P. M. Giordano, and W. C. Lindsay (eds.) Micronutrients in agriculture: Madison, Wisc., Soil Sci. Soc. Amer., 666 p.
Gardiner, J., 1974, The chemistry of cadmium in natural water, I: A study of cadmium complex formation using the cadmium specific-ion electrode: Water Res., v. 8, pp. 22–30.
Giesy, J. P., and L. A. Briese, 1977, Metals associated with organic carbon extracted from Okefenokee Swamp water: Chem. Geol., v. 20, pp. 109–120.
Giesy, J. P., and D. Paine, 1977, Effects of naturally occurring aquatic organic fractions on241Am uptake byScenedesmus obliquus (Chlorophyceae) andAeromonas hydrophyla (Pseudomonadaceae): Environ Appl. Microbiol., v. 33, pp. 89–96.
Giesy, J. P., G. J. Leversee, and D. R. Williams, 1977, Effects of naturally occurring aquatic organic fractions on cadmium toxicity toSimocephalus serrulatas (Daphnidae) andGambusia affinis (Poecillidae): Water Res., v. 11, (in press).
Giesy, J. P., D. Paine, and L. W. Hersloff, 1977, Effects of naturally occurring organics on plutonium-237 uptake by algae and bacteria, in Dunaway, P., and M. White (eds.), Transuranics in natural environments, Proceedings of a Symposium at Gatlinburg, TN, October 5–7. 1976, Nevada Applied Ecology Group, Energy Research and Development Administration. NVO178, VC2, pp. 531–543.
Hartung, R., 1973, Biological effects of heavy metal pollutants in water: Advan. Exp. Med. Biol., v. 40, pp. 161–172.
Hem, J. D., 1972, Chemistry and occurrence of cadmium and zinc in surface water and ground water: Water Resources Res., v. 8, pp. 661–679.
Hem, J. D., 1976a, Inorganic geochemistry of lead in water,in Lovering, T. G. (ed.), Lead in the environment: U.S. Geol. Survey Prof. Paper 957.
Hem, J. D., 1976b, Geochemical controls on lead concentrations in stream water and sediments: Geochim. Cosmochim. Acta, v. 40, pp. 599–609.
Hem, J. D., and W. H. Durum, 1973, Solubility and occurrence of lead in surface water: Amer. Water Works Assoc. Jour., v. 16, pp. 562–568.
Jenne, E. A., 1968, Controls on Mn, Fe, Co, Ni, Cu and Zn concentrations in soils and water: The significant role of hydrous Mn and Fe oxides: Advan. Chem. Ser., v. 73, pp. 337–388.
Jenne, E. A., and S. N. Luoma, 1975, Forms of trace elements in soil sediments and associated waters: An overview of their determination and biological availability: Biological implications of metals in the environment, 15th Life Sciences Symposium, Hanford, Sept. 27–Oct. 1.
Jones, B. F., V. C. Kennedy, and G. W. Zellweger, 1974, Comparison of observed and calculated concentrations of dissolved Al and Fe in stream water: Water Resources Res., v. 10, pp. 791–793.
Katz, F. J., 1917, Stratigraphy in southwestern Maine and southeastern New Hampshire: U.S. Geol. Survey Prof. Paper 108-I, pp. 165–177.
Kinkade, M., and H. E. Erdman, 1975, the influence of hardness components (Ca2+ and Mg2+) in water on the uptake and concentration of cadmium in a simulated freshwater system: Environ. Res., v. 10, pp. 308–313.
Koljonen, T., and L. Carlson, 1975, Behavior of the major elements and minerals in sediments of four humic lakes in southeastern Finland: Societas Geographica Fenniae, Helsinki, ISSN 0015-0010. 47 p.
Kubota, J., E. L. Mills, and R. T. Oglesby, 1974, Lead, Cd, Zn, Ca and Co in streams and lake waters of Cayuga Lake basin, New York: Environ. Sci. Technol., v. 8, pp. 243–248.
Leckie, J. O., and R. O. James, 1974, Control mechanisms for trace metals in natural waters,in Rubin, A. J. (ed.), Aqueous-environmental chemistry of metals: Ann Arbor, Mich., Ann Arbor Science, 380 p.
Leavitt, H. W., and E. H. Perkins, 1935, A survey of road materials and glacial geology of Maine: Vol. 2: Glacial geology of Maine: University Maine Press, Maine Technology Experiment Station Bulletin No. 30, pp. 118–169.
Lowenthal, R. E., and G. V. R. Marais, 1976, Carbonate chemistry of aquatic systems: Ann Arbor, Mich., Ann Arbor Sci., 433 p.
Mairs, D. F., 1966, A total alkalinity atlas for Maine lake waters: Limnol. Oceonogr., v. 11, pp. 68–72.
Manning, P. G., and S. Ramamoorthy, 1973, Equilibrium studies of metal-ion complexes of interest to natural waters, VII: Mixed-ligand complexes of Ca(II) involving fulvic acid as primary ligand: Jour. Inorg. Nucl. Chem., v. 35, pp. 1577–1581.
McGlynn, J. A., 1974, Instrumental methods for the determination of trace metals in water: Australian Water Res. Council Technical Paper, v. 8, pp. 53–78.
McKee, J. E., and H. W. Wolf, 1963, Water quality criteria: California State Res. Central Bd., Pub. No. 3-A, 548 p.
Moeller, T., 1958, Qualitative Analysis: New York, McGraw-Hill Book Co. Inc., 554 p.
Morel, F., R. E. McDuff, and J. J. Morgan, 1974, Interactions and chemostasis in aquatic systems: Role of pH, pE, solubility and complexation,in Singer, P. C. (ed.), Trace metals and metal-organic interactions in natural waters: Ann Arbor, Mich., Ann Arbor Sci., 380 p.
Oldham, W. D., and E. F. Gloyna, 1969, Effect of colored organics on iron removal: Amer. Water Wks. Assoc. Jour., v. 61, pp. 610–614.
Pagenkopf, G. K., R. C. Russo, and R. V. Thorston, 1974, Effect of complexation on toxicity of copper to fishes: Jour. Fish. Res. Bd. Can., v. 31, pp. 462–465.
Perdue, E. M., K. C. Beck, and J. H. Reuter, 1976, Organic complexes of iron and aluminum in natural waters: Nature (London), v. 260, pp. 418–420.
Pickering, Q. H., and C. Henderson, 1966, The acute toxicity of some heavy metals to different species of warm water fishes: Air Water Pollut. Int. Jour., v. 10, pp. 453–463.
Piper, D. Z., 1971, The distribution of Co, Cr, Cu, Fe, Mn, Ni and Zn in Framvaren, A Norwegian anoxic fjord: Geochim. Cosmochim. Acta, v. 35, pp. 531–550.
Pittwell, L. R., 1974, Metals coordinated by ligands normally found in natural waters: Jour. Hydrol., v. 21, pp. 301–304.
Plumb, R. H., and G. F. Lee, 1973, A note on the iron-organic relationship in natural water: Water Res., v. 7, pp. 581–585.
Ramamoorthy, S., and D. J. Kushner, 1975, Heavy metal binding sites in river water: Nature (London), v. 256, pp. 399–401.
Riffaldi, R., and R. Levi-Minzi, 1975, Adsorption and desorption of Cd on humic acid fraction of soils: Water, Air Soil Pollut., v. 5, pp. 179–184.
Schnitzer, M., and S. M. I. Skinner, 1967, Organo-metallic interactions in soils and waters,in Faust, S. D., and J. V. Hunter (eds.), Organic compounds in natural environments: New York, Marcel Decker, pp. 297–315.
Shapiro, J., 1957, Chemical and biological studies on the yellow organic acids of lake water: Limnol. Oceanogr., v. 2, pp. 161–179.
Shapiro, J., 1966, Iron available to algae: Preliminary report on a new approach to its estimation in lake water through the use of the ferrigram,in Chemical environment in the aquatic habitat: Amsterdam, Noord-Hollansche V.M., Proc. of an IBP Symp., pp. 219–228.
Shiroya, R., and K. Kumada, 1976, Combination reaction between humic acid and calcium ions: Soil Sci. Plant. Nutr., v. 22, no. 3, pp. 345–349.
Sprague, J. B., 1969, Measurement of pollutant toxicity to fish, I: Bioassay methods for acute toxicity: Water Res., v. 3, pp. 3793–3821.
Stiff, M. J., 1971, The chemical states of copper in polluted freshwater and a scheme of analysis to differentiate them: Water Res., v. 5, pp. 585–599.
Stumm, W., and H. Bilinski, 1973, Trace metals in natural waters: Difficulties of interpretation arising from our ignorance on their speciation: New York, Pergamon Press, Adv. Water Poll. Res., 6th Int. Conf., pp. 39–49.
Sunda, W. G., and J. M. Lewis, 1976, Determination of the binding of copper and cadmium in a coastal river-estuarine system using ion-selective electrodes: Beaufort, N.C., Atlantic Estuarine Fisheries Center Annual Report, pp. 53–77.
Waris, H., 1953, The significance for algae of chelating substances in the nutrient solutions: Physiol. Plant, v. 6, pp. 538–543.
Webber, W. J., and H. S. Posselt, 1974, Equilibrium models and precipitation reactions for cadmium (II)in Rubin, A. J., (ed.), Aqueous environmental chemistry of metals: Ann Arbor, Mich., Ann Arbor Sci., 390 p.
Whitfield, P. H., and A. G. Lewis, 1976, Control of biological availability of trace metals to a calanoid copepod in a coastal fjord: Estuar. Coast. Mar. Sci., v. 4, pp. 255–266.
Zitko, P., W. V. Carson, and W. G. Carson, 1973, Prediction of incipient lethal levels of copper to juvenile Atlantic salmon in the presence of humic acid by cupric electrode: Env. Cont. Tox. Bull., v. 10, pp. 265–271.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Giesy, J.P., Briese, L.A. & Leversee, G.J. Metal binding capacity of selected maine surface waters. Geo 2, 257–268 (1978). https://doi.org/10.1007/BF02430672
Issue Date:
DOI: https://doi.org/10.1007/BF02430672