Abstract
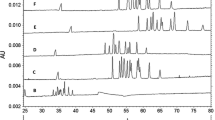
In an attempt to increase the range of analytical techniques able to monitor ultimate degradation stages of degradable, biodegradable, and bioresorbable polymers, capillary zone electrophoresis (CZE) was used to analyze tentatively oligomers formed during thermal condensation of lactic, glycolic, anddl-3-hydroxybutyric acids. The influence of the buffer and of capillary coating are discussed in terms of electroosmotic flow. Typical analyses were first performed using a 0.1M borate buffer (pH 8.9) with anodic injection. In the case of lactic acid, seven peaks were well separated, while only three peaks were observed for glycolic acid. A more complex situation was found fordl-3-hydroxybutyric acid oligomers. The first five peaks were split. The major component of each doublet was attributed to hydroxy-terminated oligomers, whereas the satellite peaks were assigned to oligomers bearing a C=C double bond at the noncarboxylic terminus. CZE of pH-sensitive lactic acid oligomers was also performed in 0.05M phosphate buffer (pH 6.8) with cathodic injection after physical coating of the fused-silica capillary with DEAE-Dextran. The buffer-soluble fraction present in lactic acid oligomers was extracted from a dichloromethane solution. Extracts issued from different batches of lactic acid condensates gave a constant water-solubility pattern whose cutoff was at the level of the decamer. CZE was also used to monitor thein vitro aging of aqueous solutions of these water-soluble oligomers. The lactyllactic acid dimer appeared more stable than higher oligomers, thus showing that ultimate stages of the degradation did not proceed at random. These physicochemical characteristics were used to complement the degradation pathway based on diffusion of oligomers duringin vitro aging of large size lactic acid plates made by compression molding. CZE data showed that lactic acid was the only component which was released in the aqueous medium during degradation.
Similar content being viewed by others
References
M. Vert, J. Feijen, A. Albertsson, G. Scott, and E. Chiellini (1992)Biodegradable Polymers and Plastics, Royal Society of Chemistry, Cambridge.
Y. Doi and K. Fukuda (1994)Biodegradable Plastics and Polymers, Elsevier, Amsterdam.
B. L. Karger, A. S. Cohen, and A. Guttman (1989)J. Chromatogr. 492, 585–614.
W. Nashabeth and Z. El Rassi (1990)J. Chromatogr. 514, 57–64.
J. Liu, O. Shirota, and M. Novotny (1991)J. Chromatogr. 559, 223–235.
J. B. L. Damm, G. T. Overklift, B. W. M. Vermeulen, C. F. Fluitsma, and G. K. van Dedem (1992)J. Chromatogr. 608, 297–309.
J. T. Smith and Z. El Rassi (1992)J. High Res. Chromatogr. 15, 573–578.
P. J. Oefner and C. Chiesa (1994)Glycobiology 4, 397–412.
C. Braud and M. Vert (1992)Polym. Bull. 29, 177–183.
J. Bullock (1993)J. Chromatogr. 645, 169–177.
R. Kuhn and S. Hoffsteter-Kuhn (1993)Capillary Electrophoresis: Principles and Practice, Springer-Verlag, Berlin.
F. Foret, L. Krivankova, and P. Boceck (1993)Capillary Zone Electrophoresis, VCH, Weinheim.
C. Vidil, C. Braud, H. Garreau, and M. Vert (1995)J. Chromatogr. 711, 323–329.
S. M. Li, H. Garreau, and M. Vert (1990)J. Mater. Sci. Mater. Med. 1, 123–130.
I. Grizzi, H. Garreau, S. Li, and M. Vert (1995)Biomaterials 16, 305–311.
S. Li and M. Vert (1995) in G. Scott and D. Gilead (Eds.),Degradable Polymers—Principles and Applications, Chapman and Hall, London, pp. 43–87.
M. Vert, F. Chabot, J. Leray, and P. Christel (1978) French Patent application No. 78-29978.
M. P. Richards and P. J. Aagaard (1994)J. Cap. Elec. 1, 90–95.
N. Cohen and E. Grushka (1994)J. Cap. Elec. 1, 112–115.
G. Schomburg, D. Belder, M. Gilges, and S. Motsch (1994)J. Cap. Elec. 1, 219–230.
C. H. Holten (1971)Lactic Acid—Properties and Chemistry of Lactic Acid and Derivatives, Verlag Chemie, Weinheim.
J. Mauduit (1991) Ph.D. thesis, Rouen, France.
D. W. McLellan and P. J. Halling (1988)J. Chromatogr. 445, 251–257.
S. Karlsson, C. Sares, R. Renstad, and A.-C. Albertsson (1994)J. Chromatogr. 669, 97–102.
S. Terabe and T. Isemura (1990)J. Chromatogr. 515, 667–676.
M. Vert, A. Torres, S. M. Li, S. Roussos, and H. Garreau (1994) in Y. Doy and K. Fukuda (Eds.),Biodegradable Plastics and Polymers, Elsevier, Amsterdam, pp. 11–23.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Braud, C., Devarieux, R., Garreau, H. et al. Capillary electrophoresis to analyze water-soluble oligo(hydroxyacids) issued from degraded or biodegraded aliphatic polyesters. J Environ Polym Degr 4, 135–148 (1996). https://doi.org/10.1007/BF02067448
Issue Date:
DOI: https://doi.org/10.1007/BF02067448