Abstract
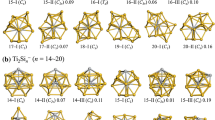
The structural, electronic and magnetic properties of Ti13, Ti12C, V13 and V12C clusters with octahedral (Oh), decahedral (D5h) and icosahedral (Ih) geometries have been analyzed by means of DFT calculations as well as the adsorption of some chemical species has been performed (12CO and 12NO) onto Ti13 (Ih) and Ti12C (Ih) clusters. The geometry energetically preferred is the icosahedron in both cases: pristine and carbide (doped with one carbon atom), for titanium and vanadium elements. The doping effect generates a transition on electronic behavior from semiconductor to metallic with respect to pristine systems for titanium clusters, whereas the vanadium clusters exhibit opposite behavior from metallic to semiconductor. The multiplicities (M = 2s + 1) remain without changes from pristine to carbide systems (excepting for octahedron), however for vanadium clusters, there is a transition M = 2 → 1 due to substitution of central atom of these systems with respect to pristine cases. The chemisorption of nitrogen monoxide onto the Ti13 (Ih) and Ti12C (Ih) clusters is around of two times stronger than carbon monoxide due to the high electronic charge transference from the titanium atoms of cluster surface toward the nitrogen atoms of NO molecules.









Similar content being viewed by others
References
Alonso JA (2000) Electronic and atomic structure, and magnetism of transition-metal clusters. Chem Rev 100(2):637–678
Bergeron DE, Castleman AW Jr, Morisato T, Khanna SN (2004) Formation and properties of halogenated aluminum clusters. J Chem Phys 121:10456–10466
Bernstein ER (2015) Neutral cluster mass spectrometry. Int J Mass Spectrom 377:248–262
Bora PL, Ahmad R, Singh AK (2015) Remarkable enhancement in hydrogen storage on free-standing Ti3B and BC3 supported Ti3 clusters. Int J Hydrogen Energy 40:1054–1061
Castro M, Liu SR, Zhai HJ, Wang LS (2003) Structural and electronic properties of small titanium clusters: a density functional theory and anion photoelectron spectroscopy study. J Chem Phys 118:2116–2123
Delley B (1990a) An all-electron numerical method for solving the local density functional for polyatomic molecules. J Chem Phys 92:508–517
Delley B (1990b) An all-electron numerical method for solving the local density functional for polyatomic molecules. J Chem Phys 92:508
Delley B (1991) Analytic energy derivatives in the numerical local-density-functional approach. J Chem Phys 94:7245–7250
Didziulis SV, Butcher KD, Perry SS (2003) Small cluster models of the surface electronic structure and bonding properties of titanium carbide, vanadium carbide, and titanium nitride. Inorg Chem 42:7766–7781
Fernando A, Weerawardene KD, Karimova NV, Aikens CM (2015) Quantum mechanical studies of large metal, metal oxide, and metal chalcogenide nanoparticles and clusters. Chem Rev 115(12):6112–6216
Furrer A, Waldmann O (2013) Magnetic cluster excitations. Rev Mod Phys 85:367
Gueorguinev GK, Pacheco JM (2003) Shapes of cage-like metal carbide clusters: first principles calculations. Phys Rev B 68:241410(R)
Hohenberg P, Kohn W (1964) Inhomogeneous electron gas. Phys Rev 136(3B):B864–B871
Huber KP, Herzberg G (1979) Molecular spectra and molecular structure. Van Nostrand, Toronto
Iyengar SS, Schlegel HB, Millam JM, Voth GA, Scuseria GE, Frisch MJ (2001) Ab initio molecular dynamics: propagating the density matrix with Gaussian orbitals. II. Generalizations based on mass-weighting, idempotency, energy conservation and choice of initial conditions. J Chem Phys 115:10291–10302
Jenaa P, Castleman AW (2015) Mass spectrometry and its role in advancing cluster science. Int J Mass Spectrom 377:235–247
Kalemos A, Mavridis A (2002) Theoretical Investigation of titanium carbide, TiC: X3Σ + , a1Σ + , A3∆, and b1∆ States. J Phys Chem A 106:3905–3908
Knappenberger KL Jr, Jones CE Jr, Sobhy MA, Iordanov I, Sofo J, Castleman AW Jr (2006) Anion photoelectron spectroscopy and density functional investigation of vanadium carbide clusters. J Phys Chem A 110:12814–12821
Largo L, Cimas A, Redondo P, Rayon VM, Barrientos C (2006) Structure of small TiCn clusters: a theoretical study. Chem Phys 330:431–440
Largo L, Cimas A, Redondo P, Rayon VM, Barrientos C (2007) Charged titanium-doped carbon clusters: structures and energetics. Int J Mass Spectrom 266:50–61
Lazauskas T, Sokol AA, Buckeridge J, Catlow CR, Escher SG, Farrow MR, Mora-Fonz D, Blum VW, Phaahla TM, Chauke HR, Ngoepe PE, Woodley SM (2018) Thermodynamically accessible titanium clusters TiN, N = 2–32. Phys Chem Chem Phys 20:13962–13973
Lee B, Lee GW (2007) Comparative study of Ti and Ni clusters from first principles. J Chem Phys 127:164316-1–164316-5
Lee C, Yang W, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Lian L, Su CX, Armentrout PB (1992) Collision-induced dissociation of Tin (n = 2–22) with Xe: bond energies, geometric structures, and dissociation pathways. J Chem Phys 97:4084–4093
Liu SR, Zhai HJ, Castro M, Wang LS (2003) Photoelectron spectroscopy of Tin clusters (n = 1-130). J Chem Phys 118:2108–2115
Martinez JI, Castro A, Rubio A, Alonso JA (2006) Photoabsorption spectra of Ti8C12 metallocarbohedrynes: theoretical spectroscopy within time-dependent density functional theory. J Chem Phys 125:074311
May BD, Cartier SF, Castleman AW Jr (1995) Delayed ionization and delayed atomic ion emission of Ti and V metallocarbohedrenes. Evidence for collective electronic effects. Chem Phys Lett 242:265–272
Medina J, De Coss R, Tapia A, Canto G (2010) Structural, energetic and magnetic properties of small Tin (n = 2–13) clusters: a density functional study. Eur Phys J B 76:427–433
Muñoz J, Pujol C, Bo C, Poblet JM, Rohmer MM, Benard M (1997) DFT description of binary metal met-cars TixZryC12 (x + y=8) and of some conformers of the M6C12, M7C12, and M8C13 clusters (M = Ti, Zr). J Phys Chem A 101:8345–8350
Muñoz J, Rohmer MM, Benard M, Bo C, Poblet JM (1999) The structure and growth mechanism of small titanium carbide clusters: a competition between C2 and C4 carbon chains. J Phys Chem A 103:4762–4768
Pakiari AH, Salarhaji M (2018) Introducing nano-particle-type properties of Tin (n = 2–6) clusters. J Mol Graph Model 85:294–303
Patzschke M, Sundholm D (2005) Density-functional-theory studies of the infrared spectra of titanium carbide nanocrystals. J Phys Chem B 109:12503–12508
Perdew JP, Wang Y (1992) Accurate and simple analytic representation of the electron-gas correlation energy. Phys Rev B 45:13244
Perdew JP, Burke K, Ernzerhof M (1997) Generalized gradient approximation made simple. Phys Rev Lett 77:3865
Ratsch C, Fielicke A, Kirilyuk A, Behler J, von Helden G, Meijer G, Scheffler M (2005) Structure determination of small vanadium clusters by density-functional theory in comparison with experimental far-infrared spectra. J Chem Phys 122:124302
Redondo P, Barrientos C, Largo A (2007) Structures and stabilities of non-linear VCn+/− (n = 1–8) clusters. Int J Mass Spectrom 263:101–112
Ryeng H, Gropen O, Swang O (1997) Molecular structure and C–O stretch frequencies of the cobalt carbonyls Co(CO)n, n = 1, 4, as studied by density functional theory. J Phys Chem A 101:8956–8958
Sakurai M, Watanabe K, Sumiyama K, Suzuki K (1999) Magic numbers in transition metal (Fe, Ti, Zr, Nb, and Ta) clusters observed by time-of-flight mass spectrometry. J Chem Phys 111:235
Salazar-Villanueva M, Hernández Tejeda PH, Pal U, Rivas-Silva JF, Rodríguez Mora JI, Ascencio JA (2006) Stable Tin (n = 2–15) clusters and their geometries: DFT calculations. J Phys Chem A 110:10274–10278
Salazar-Villanueva M, Tejeda PHH, Rivas-Silva JF, Ascencio JA (2008) Stability and physicochemical principles for icosahedral Ti12X (X = Li to Xe) clusters: a DFT study. J Nanosci Nanotech 8(5):2475–2478
Sato Y, Yumura T, Suenaga K, Moribe H, Nishide D, Ishida M, Shinohara H, Iijima S (2006) Direct imaging of intracage structure in titanium–carbide endohedral metallofullerene. Phys Rev B 73:193401
Schlegel HB, Millam JM, Iyengar SS, Voth GA, Daniels AD, Scuseria GE, Frisch MJ (2001) Ab initio molecular dynamics: propagating the density matrix with Gaussian orbitals. J Chem Phys 114:9758–9763
Schlegel HB, Iyengar SS, Li X, Millam JM, Voth GA, Scuseria GE, Frisch MJ (2002) Ab initio molecular dynamics: propagating the density matrix with Gaussian orbitals. II. Generalizations based on mass-weighting, idempotency, energy conservation and choice of initial conditions. J Chem Phys 117:8694–8704
Sun H, Ren Y, Wang G (2001) Structural, electronic, and magnetic properties of mixed V13-xRhx (x = 0 to 13) clusters. Phys Stat Sol (b) 225(2):301–310
Sun H, Zhang W, Ning X (2018) Density functional calculation of structural and electronic properties of Tin-xAlx (n = 2–8, 13, x = 0–n) clusters. J Phys Chem Solids 118:126–136
Tono K, Terasaki A, Ohta T, Kondow T (2002) Geometric and electronic structures of V2C2− and V2C2 studied by photoelectron spectroscopy and density-functional calculations. Chem Phys Lett 351:135–141
Verlet L (1967) Computer experiments on classical fluids I Thermodynamical properties of Lennard-Jones molecules. Phys Rev 159:98
Wang SY, Duan W, Wang CY (2002) First-principles investigation into the structural stability of icosahedral Ti12X clusters (X = B, C, N, Al, Si, P, V, Cr, Mn, Fe, Co and Ni). J Phys B At Mol Opt Phys 35:4015
Wu G, Yang M, Guo X, Wang J (2012) Comparative DFT study of N2 and NO adsorption on vanadium clusters Vn (n = 2–13). J Comput Chem 33:1854–1861
Xing L, Akasaka T, Nagase S (2013) Carbide cluster metallofullerenes: structure, properties, and possible origin. Acc Chem Res 46(7):1627–1635
Zhang H, Wu H, Geng L, Jia Y, Yang M, Luo Z (2019) Phys Chem Chem Phys 21:11234–11241
Zhao JJ, Qiu Q, Wang BL, Wang JL, Wang GH (2001) Geometric and electronic properties of titanium clusters studied by ultrasoft pseudopotential. Solid State Commun 118:157–161
Zhao Y, Dillon AC, Kim YH, Heben MJ, Zhang SB (2006) Self-catalyzed hydrogenation and dihydrogen adsorption on titanium carbide nanoparticles. Chem Phys Lett 425:273–277
Acknowledgements
MSV and ABH thank VIEP-BUAP and PROMEP Mexico for supporting this study. We also acknowledge the computational resources provided by CNS-Ipicyt, Mexico. The authors gratefully acknowledge the computational resources, technical expertise, and support provided by the Laboratorio Nacional de Supercómputo del Sureste de México and the CONACYT network of national laboratories.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hernandez, A.B., Cortés-Arriagada, D., García, H.C. et al. Quantum molecular study on doping effect in titanium and vanadium clusters: their application to remove some chemical species. Appl Nanosci 10, 37–49 (2020). https://doi.org/10.1007/s13204-019-01072-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-019-01072-8