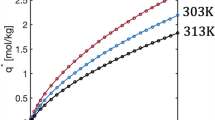
Abstract
Several theoretical and empirical models are available to correlate experimental breakthrough curves of water pollutants, one of which is the two-parameter Belter model. Although not as well known as the century-old Bohart–Adams model, the Belter model is being used with sufficient frequency to merit wider awareness of its strengths and weaknesses. Through a systematic analysis, it is shown that the two adjustable parameters of the Belter model are analogous to the equilibrium capacity and rate parameters of the Bohart–Adams model. Breakthrough curves predicted by the Belter model are perfectly symmetric because their inflection points are invariant and always correspond to the midpoint of the curves. As a consequence, the Belter model provides poor fits to asymmetric breakthrough curves. In this work, an improved version of the Belter model is introduced. The new model with a floating inflection point manifests excellent conformity with mildly and, more importantly, severely asymmetric breakthrough curves.








Similar content being viewed by others
References
Apiratikul, R., & Chu, K. H. (2021). Improved fixed bed models for correlating asymmetric adsorption breakthrough curves. Journal of Water Process Engineering, 40, 101810.
Belter, P. A., Cussler, E. L., & Hu, W.-S. (1988). Bioseparations: Downstream processing for biotechnology. John Wiley & Sons.
Bohart, G. S., & Adams, E. Q. (1920). Some aspects of the behavior of charcoal with respect to chlorine. Journal of the American Chemical Society, 42, 523–529.
Brady, J. M., Tobin, J. M., & Roux, J.-C. (1999). Continuous fixed bed biosorption of Cu2+ ions: Application of a simple two parameter mathematical model. Journal of Chemical Technology and Biotechnology, 74, 71–77.
Chatterjee, A., & Schiewer, S. (2011). Biosorption of cadmium(II) ions by citrus peels in a packed bed column: Effect of process parameters and comparison of different breakthrough curve models. Clean-Soil Air Water, 39, 874–881.
Chu, K. H. (2020). Breakthrough curve analysis by simplistic models of fixed bed adsorption: In defense of the century-old Bohart-Adams model. Chemical Engineering Journal, 380, 122513.
Da Costa Rocha, A. C., Scaratti, G., Moura-Nickel, C. D., da Silva, T. L., Gurgel Adeodato Vieira, M., Peralta, R. M., Peralta, R. A., de Noni Jr, A., & Peralta Muniz Moreira, R. d. F. . (2020). Economical and technological aspects of copper removal from water using a geopolymer and natural zeolite. Water, Air, & Soil Pollution, 231, 361.
Faisal, A. A. H., Ali, I. M., Naji, L. A., Madhloom, H. M., & Al-Ansari, N. (2020). Using different materials as a permeable reactive barrier for remediation of groundwater contaminated with landfill’s leachate. Desalination and Water Treatment, 175, 152–163.
Faisal, A. A. H., Jasim, H. K., Naji, L. A., Naushad, Mu., & Ahamad, T. (2021). Cement kiln dust-sand permeable reactive barrier for remediation of groundwater contaminated with dissolved benzene. Separation Science and Technology, 56, 870–883.
Fernandez, M. E., Nunell, G. V., Bonelli, P. R., & Cukierman, A. L. (2014). Activated carbon developed from orange peels: Batch and dynamic competitive adsorption of basic dyes. Industrial Crops and Products, 62, 437–445.
Ghasemi, M., Keshtkar, A. R., Dabbagh, R., & Safdari, S. J. (2011). Biosorption of uranium(VI) from aqueous solutions by Ca-pretreated Cystoseira indica alga: Breakthrough curves studies and modeling. Journal of Hazardous Materials, 189, 141–149.
Khoo, E.-C., Ong, S.-T., & Ha, S.-T. (2012). Removal of basic dyes from aqueous environment in single and binary systems by sugarcane bagasse in a fixed-bed column. Desalination and Water Treatment, 37, 215–222.
Knapik, E., Chruszcz-Lipska, K., Stopa, J., Marszałek, M., & Makara, A. (2020). Separation of BTX fraction from reservoir brines by sorption onto hydrophobized biomass in a fixed-bed-column system. Energies, 13, 1064.
Lee, C. K., Ong, S. T., & Zainal, Z. (2008). Ethylenediamine modified rice hull as a sorbent for the removal of Basic Blue 3 and Reactive Orange 16. International Journal of Environmental Pollution, 34, 246–260.
Lee, C.-G., Kim, J.-H., Kang, J.-K., Kim, S.-B., Park, S.-J., Lee, S.-H., & Choi, J.-W. (2015). Comparative analysis of fixed-bed sorption models using phosphate breakthrough curves in slag filter media. Desalination and Water Treatment, 55, 1795–1805.
Lodeiro, P., Herrero, R., & de Vicente, M. E. S. (2006). The use of protonated Sargassum muticum as biosorbent for cadmium removal in a fixed-bed column. Journal of Hazardous Materials, B137, 244–253.
Mouldar, J., Hatimi, B., Hafdi, H., Joudi, M., Belghiti, M. E. A., Nasrellah, H., El Mhammedi, M. A., El Gaini, L., & Bakasse, M. (2020). Pyrrhotite ash waste for capacitive adsorption and fixed-bed column studies: Application for reactive red 141 dye. Water, Air, & Soil Pollution, 231, 205.
Naji, L. A., Faisal, A. A. H., Rashid, H. M., Naushad, Mu., & Ahamad, T. (2020). Environmental remediation of synthetic leachate produced from sanitary landfills using low-cost composite sorbent. Environmental Technology & Innovation, 18, 100680.
Ramirez, C.M., da Silva, M. P., Ferreira, L. S. G. & Vasco, E. O. (2007). Mathematical models applied to the Cr(III) and Cr(VI) breakthrough curves. Journal of Hazardous Materials, 146, 86–90.
Riazi, M., Keshtkar, A. R., & Moosavian, M. A. (2016). Biosorption of Th(IV) in a fixed-bed column by Ca-pretreated Cystoseira indica. Journal of Environmental Chemical Engineering, 4, 1890–1898.
Ruthven, D. M. (1984). Principles of adsorption and adsorption processes. John Wiley & Sons.
Saldaña-Robles, A., Damian-Ascencio, C. E., Guerra-Sanchez, R. J., Saldaña-Robles, A. L., Saldaña-Robles, N., Gallegos-Muñoz, A., & Cano-Andrade, S. (2018). Effects of the presence of organic matter on the removal of arsenic from groundwater. Journal of Cleaner Production, 183, 720–728.
Sigrist, M. E., Beldomenico, H. R., Tarifa, E. E., Pieck, C. L., & Vera, C. R. (2011). Modelling diffusion and adsorption of As species in Fe/GAC adsorbent beds. Journal of Chemical Technology and Biotechnology, 86, 1256–1264.
Srinivasan, A., & Viraraghavan, T. (2014). Oil removal in a biosorption column using immobilized M. rouxii biomass. Desalination and Water Treatment, 52, 3085–3095.
Stanley, L. C., & Ogden, K. L. (2003). Biosorption of copper (II) from chemical mechanical planarization wastewaters. Journal of Environmental Management, 69, 289–297.
Sulaymon, A. H., Ebrahim, S. E., Abdullah, S. M., & Al-Musawi, T. J. (2010). Removal of lead, cadmium, and mercury ions using biosorption. Desalination and Water Treatment, 24, 344–352.
Sulaymon, A. H., Faisal, A. A. H., & Khaliefa, Q. M. (2015). Cement kiln dust (CKD)-filter sand permeable reactive barrier for the removal of Cu(II) and Zn(II) from simulated acidic groundwater. Journal of Hazardous Materials, 297, 160–172.
Teng, M.-Y., & Lin, S.-H. (2006). Removal of basic dye from water onto pristine and HCl-activated montmorillonite in fixed beds. Desalination, 194, 156–165.
Thomas, H. C. (1944). Heterogeneous ion exchange in a flowing system. Journal of the American Chemical Society, 66, 1664–1666.
Tovar-Gómez, R., Moreno-Virgen, M. R., Dena-Aguilar, J. A., Hernández-Montoya, V., Bonilla-Petriciolet, A., & Montes-Morán, M. A. (2013). Modeling of fixed-bed adsorption of fluoride on bone char using a hybrid neural network approach. Chemical Engineering Journal, 228, 1098–1109.
Wong, K. K., Lee, C. K., Low, K. S., & Haron, M. J. (2003). Removal of Cu and Pb from electroplating wastewater using tartaric acid modified rice husk. Process Biochemistry, 39, 437–445.
Xiu, G.-H., Nitta, T., Li, P., & Jin, G. (1997). Breakthrough curves for fixed-bed adsorbers: Quasi-lognormal distribution approximation. American Institute of Chemical Engineers Journal, 43, 979–985.
Yoon, Y. H., & Nelson, J. A. (1984). Application of gas adsorption kinetics. I. A theoretical model for respirator cartridge service life. American Industrial Hygiene Association Journal, 45, 509–516.
Funding
The author received no specific funding for writing this article.
Availability of dataAll data analyzed during this study are included in this published article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares he has no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chu, K.H. A Modified Belter Model for Correlating Asymmetric Breakthrough Curves of Water Pollutants. Water Air Soil Pollut 232, 451 (2021). https://doi.org/10.1007/s11270-021-05399-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05399-3