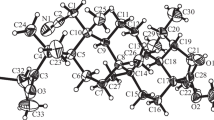
Abstract
Epoxidation of 2-cyano-2,3-seco-24-nor-4(23)-ene derivatives of oleanolic acid with m-chloroperoxybenzoic acid, followed by treatment of the resulting epoxides with boron trifluoride–diethyl ether complex, afforded new 24-noroleanane derivatives whose structure was determined using two-dimensional NMR correlation techniques (1H–1H COSY, 1H–1H NOESY, 1H–13C HSQC, 1H–13C HMBC). Depending on the conditions, the reaction of methyl 2-cyano-2,3-seco-24-norolean-4(23)-ene-28-oate with m-chloroperoxybenzoic acid may be regioselective with the formation of 4(23)-epoxy or 12-oxo derivative, as well as allylic oxidation product of the isopropenyl moiety.




Similar content being viewed by others
REFERENCES
Chen, Y., Liu, J., Yang, X., Zhao, X., and Xu, H., J. Pharm. Pharmacol., 2005, vol. 57, p. 259. https://doi.org/10.1211/0022357055407
Ayeleso, T.B., Matumba, M., and Mukwevho, E., Molecules, 2017, vol. 22, p. 1915. https://doi.org/10.3390/molecules22111915
Somova, L.O., Nadar, A., Rammanan, P., and Shode, F.O., Phytomedicine, 2003, vol. 10, p. 115. https://doi.org/10.1078/094471103321659807
Chen, S., Wen, X., Zhang, W., Wang, C., Liu, J., and Liu, C., FASEB J., 2017, vol. 31, p. 1085. https://doi.org/10.1096/fj.201601022R
Wu, H., Zhong, Q., Zhong, R., Huang, H., Xia, Z., Ke, Z., Zhang, Z., Song, J., and Jia, X., Int. J. Nanomed., 2016, vol. 11, p. 6337. https://doi.org/10.2147/IJN.S119839
Li-Ting, T., Long, M., and Nian-Sheng, D., China J. Chin. Mater. Med., 2002, vol. 12, p. 9.
Jeong, D.W., Kim, Y.H., Kim, H.H., Ji, H.Y., Yoo, S.D., Choi, W.R., Lee, S.M., Han, C.-K., and Lee, H.S., Biopharm. Drug Dispos., 2007, vol. 28, p. 51. https://doi.org/10.1002/bdd.530
Liby, K., Royce, D.B., Williams, C.R., Risingsong, R., Yore, M.M., Honda, T., Gribble, G.W., Dmitrovsky, E., Sporn, T.A., and Sporn, M.B., Cancer Res., 2007, vol. 67, p. 2414. https://doi.org/10.1158/0008-5472.CAN-06-4534
To, C., Ringelberg, C.S., Royce, D.B., Williams, C.R., Risingsong, R., Sporn, M.B., and Liby, K.T., Carcinogenesis, 2015, vol. 36, p. 769. https://doi.org/10.1093/carcin/bgv061
Fu, L.-F., Lin, Q.-X., Onyango, E.O., Liby, K.T., Sporn, M.B., and Gribble, G.W., Org. Biomol. Chem., 2017, vol. 15, p. 6001. https://doi.org/10.1039/C7OB01420A
Kazakova, O.B., Kazakov, D.V., Yamansarov, E.Yu., Medvedeva, N.I., Tolstikov, G.A., Suponitsky, K.Yu., and Arkhipov, D.E., Tetrahedron Lett., 2011, vol. 52, p. 976. https://doi.org/10.1016/j.tetlet.2010.12.047
Husnutdinova, E.F., Lobov, A.N., Kukovinets, O.S., Kazakova, O.B., and Kataev, V.E., Russ. J. Org. Chem., 2015, vol. 51, p. 261. https://doi.org/10.1134/S1070428015020219
Khusnutdinova, E.F., Medvedeva, N.I., Kazakov, D.V., Kukovinets, O.S., Lobov, A.N., Suponitsky, K.Yu., and Kazakova, O.B., Tetrahedron Lett., 2016, vol. 57, p. 148. https://doi.org/10.1016/j.tetlet.2015.11.086
Khusnutdinova, E.F., Kazakova, O.B., Lobov, A.N., Kukovinets, O.S., Suponitsky, K.Yu., Meyers, C.B., and Prichard, M.N., Org. Biomol. Chem., 2019, vol. 17, p. 585. https://doi.org/10.1039/C8OB02624F
Finlay, H.J, Honda, T., Gribble, G.W., Danielpour, D., Benoit, N.E., Suh, N., Williams, C., and Sporn, M.B., Bioorg. Med. Chem. Lett., 1997, vol. 7, p. 1769. https://doi.org/10.1016/S0960-894X(97)00310-7
Bednarczyk-Cwynar, B., Ruszkowski, P., Bobkiewicz-Kozlowska, T., and Zaprutko, L., Anti-Cancer Agents Med. Chem., 2016, vol. 16, p. 579. https://doi.org/10.2174/1871520615666150907095756
Bednarczyk-Cwynar, B., Partyka, D., and Zaprutko, L., PLoS One, 2015, vol. 10, article ID e0122857. https://doi.org/10.1371/journal.pone.0122857
Rao, L.K., Ramraj, S.K., and Sundararamaiah, T., J. Indian Chem. Soc., 1980, vol. 57, p. 833.
Cohen, K.F., Kazlauskas, R., and Pinhey, J.T., J. Chem. Soc., Perkin Trans. 1, 1973, p. 2076. https://doi.org/10.1039/p19730002076
Kazakova, O.B., Khusnutdinova, E.F., Lobov, A.N., Medvedeva, N.I., and Spirikhin, L.V., Chem. Nat. Compd., 2011, vol. 47, p. 579. https://doi.org/10.1007/s10600-011-9999-9
Klinot, J., Hovorková, N., and Vystrčil, A., Collect. Czech. Chem. Commun., 1970, vol. 35, p. 1105. https://doi.org/10.1135/cccc19701105
Chrobak, E., Bebenek, E., Marciniec, K., Kadela-Tomanek, M., Siudak, S., Latocha, M., and Boryczka, S., J. Mol. Struct., 2021, vol. 1226, article ID 129394. https://doi.org/10.1016/j.molstruc.2020.129394
Funding
This study was performed in the framework of state assignment (project nos. AAAA-A20-120012090023-8, AAAA-A20-120012090029-0).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Additional information
Translated from Zhurnal Organicheskoi Khimii, 2021, Vol. 57, No. 9, pp. 1252–1259 https://doi.org/10.31857/S0514749221090032.
Rights and permissions
About this article
Cite this article
Zakirova, L.M., Tretyakova, E.V., Baikova, I.P. et al. Synthesis of 24-Noroleanolic Acid Derivatives. Russ J Org Chem 57, 1405–1411 (2021). https://doi.org/10.1134/S1070428021090037
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428021090037