Abstract
Density measurements have been carried out on the melt system diopside-anorthite from room temperature to 1600° C at 1 atm, and from 1400° C to 1800° C at pressures up to 20 Kb. The densities were determined based on the dilatometric curve and density at 22° C for lowtemperatures, the double-bob Archimedean method for high-temperatures at 1 atm, and on the sinking and floating spheres method for high-pressure conditions.
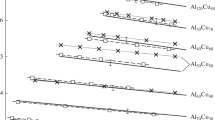
The results at 1 atm indicate that the thermal expansion coefficient of the glassy state is almost constant, while that of the liquid state decreases with increasing temperature. Density decreased with increasing anorthite content for both glassy and liquid states. Melts in the liquid-state mix ideally with respect to volume, while the glassy state exhibits a maximum excess volume at Di30An70. Density-pressure relations clearly show a density reversion between diopsiderich and anorthite-rich melts; the anorthite-rich melt becomes denser than diopside-rich melt at pressures above 8 kb.
The free volumes of both the liquid and glassy states decreased with increasing anorthite content.
Isothermal compressibilities and the hard-sphere diameter have been calculated based on the hard-sphere liquid model using thermal expansion coefficients and surface tension data. Calculated compressibilities for diopside-rich melt (Di:>Di60) agreed well with the experimental data, while calculated and observed compressibilities for anorthite-rich melt did not. This evidence indicates that diopside melt may be regarded as a discrete-melt composed of small constituent units (about 10 Å in average diameter) and much interstitial space, while anorthite melt is a three-dimensional network melt with little interstitial space. The critical composition Di60An40 is similar to that of the eutectic and corresponds to breaks between composition and other physical properties. It is proposed that the composition may reflect a kind of “critical state” in the substitution of the “continuous structure” of anorthite melt for the “discrete structure” of diopside melt. The critical state may be interpreted based on the site-percolation theory.
Similar content being viewed by others
References
Arndt J, Häberle F (1973) Thermal expansion and glass transition temperatures of synthetic glasses of plagioclase-like compositions. Contrib Mineral Petrol 39:175–183
Berman H, Daly RA, Spicer HC (1942) Density at room temperature and 1 atmosphere. In: Birch F, Schairer JF, Spicer HC (eds) Handbook of physical constants, No. 36. Geol Soc Am Spec Pap, pp 7–26
Binsted N, Greaves GN, Henderson CMB (1985) An EXAFS study of glassy and crystalline phases of compositions CaAl2Si2O8 (anorthite) and CaMgSi2O6 (diopside). Contrib Mineral Petrol 89:103–109
Birch F (1961) The velocity of compressional waves in rocks to 10 kilobars. J Geophys Res 66:2199–2224
Bondi A (1954) Free volumes and free rotation in simple liquids and liquid saturated hydrocarbons. J Phys Chem 58:929–939
Boyer AJG, Fray DJ, Meadowcroft TR (1967) Application of the rigid sphere model to the pure molten phosphates of calcium, sodium, and zinc. Phys Chem Glasses 8:96–100
Brearley M, Dickinson JE Jr, Scarfe CM (1986) Pressure dependence of melt viscosities on the join diopside-albite. Geochim Cosmochim Acta 50:2563–2570
Cranmer D, Uhlmann DR (1981) Viscosities in the system albiteanorthite. J Geophys Res 86:7951–7956
Cukierman M, Uhlmann DR (1973) Viscosity of liquid anorthite. J Geophys Res 78:4920–4923
Dane EB Jr (1942) Density at high temperature; Thermal expansion In: Birch F, Schairer JF, Spicer HC (ed) Handbook of physical constants, No. 36. Geol Soc Am Spec Pap, pp 30–37
Daněk V, Ličko T (1981) Apparatus for the measurement of physico-chemical properties of oxide melts. Silikaty 25:153–164 (in Czechoslovak)
Dunn T (1982) Oxygen diffusion in three silicate melts along the join diopside-anorthite. Geochim Cosmochim Acta 46:2293–2299
Finger LW, Ohashi Y (1976) The thermal expansion of diopside to 800° C and a refinement of the crystal structure at 700° C. Am Mineral 61:303–310
Foit FF Jr, Peacor DR (1973) The anorthite crystal structure at 410 and 830° C. Am Mineral 58:665–675
Fray DJ (1970) The structure of alkali silicate melts. Phys Chem Glasses 11:219–222
Fujii T, Kushiro I (1977) Density, viscosity, and compressibility of basaltic liquid at high pressures. Carnegie Inst Washington Yearb 76:419–424
Hummel W, Arndt J (1985) Variation of viscosity with temperature and composition in the plagioclase system. Contrib Mineral Petrol 90:83–92
Kirkpatrick RJ (1974) Kinetics of crystal growth in the system CaMgSi2O6-CaAl2SiO6. Am J Sci 274:215–242
Kirkpatrick RJ, Oestrike R, Weiss CA Jr, Smith KA, Oldfield E (1986) High-resolution 27A1 and 29Si NMR spectroscopy of glasses and crystals along the join CaMgSi2O6-CaAl2SiO6. Am Mineral 71:705–711
Kushiro I (1976) Changes in viscosity and structure of melt of NaAlSi2O6 composition at high pressures. J Geophys Res 81:6347–6350
Kushiro I (1982) Density of tholeiite and alkali basalt magmas at high pressures. Carnegie Inst Washington Yearb 81:305–309
Lange RA, Carmichael ISE (1987) Densities of Na2O-K2O-CaO-MgO-FeO-Fe2O3-Al2O3-TiO2-SiO2 liquids: New measurements and derived partial molar properties. Geochim Cosmochim Acta 51:2931–2946
Levien L, Prewitt CT (1981) High-pressure structural study of diopside. Am Mineral 66:315–323
Ličko T, Daněk V (1982) Densities of melts in the system CaSiO3-CaMgSi2O6-Ca2MgSi2O7. Physics Chem Glasses 23:67–71
Matson DW, Sharma SV, Philpotts JA (1986) Raman spectra of some tectosilicates and of glasses along the orthoclase-anorthite and nepheline-anorthite joins. Am Mineral 71:694–704
Matsui Y, Kawamura K (1984) Computer simulation of structures of silicate melts and glasses. In: Sunagawa I (ed) Materials science of the earth's interior, Terra Scientific Publications, Tokyo, pp 3–23
Mayer SW (1963) A molecular parameter relationship between surface tension and liquid compressibility. J Phys Chem 67:2160–2164
McMillan P (1984) A raman spectroscopic study of glasses in the system CaO-MgO-SiO2. Am Mineral 69:645–659
Mo X, Carmichael ISE, Rivers ML, Stebbins JF (1982) The partial molar volume of Fe2O3 in multicomponent silicate liquids and the pressure dependence of oxygen fugacity in magmas. Mineral Mag 45:237–245
Murase T, McBirney AR (1973) Properties of some common igneous rocks and their melts at high temperature. Geol Soc Am Bull 84:3563–3592
Murayama K (1987) Amorphous and fractal. In Fractal science, Asakura, Tokyo, pp 170–207 (in Japanese)
Murdoch JB, Stebbins JF, Carmichael ISE (1985) High-resolution 29Si NMR study of silicate and aluminosilicate glasses: the effect of network-modifying cations. Am Mineral 70:332–343
Mysen BO, Virgo D, Scarfe CM (1980) Relations between the anionic structure and viscosity of silicate melts — a Raman spectroscopic study. Am Mineral 65:690–710
Mysen BO, Virgo D, Danckwerth P, Seifert FA, Kushiro I (1983) Influence of pressure on the structure of melts on the joins NaAlO2-SiO2, CaAl2O4-SiO2, and MgAl2O4-SiO2. Neues Jahrb Mineral Abh 147:281–303
Nelson SA, Carmichael ISE (1979) Partial molar volumes of oxide components in silicate liquids. Contrib Mineral Petrol 71:117–124
Ogino K, Nishiwaki J (1976) Density and electrical conductivity of slags in the system CaO-SiO2-Al2O3. Molten Salt Committee Meeting, Abstract:31–32 (in Japanese)
Ohtani E (1984) Generation of komatiite magma and gravitational differentiation in the deep upper mantle. Earth Planet Sci Lett 67:261–272
Okuno M, Marumo F, Ossaka J, Ishizawa N, Minato I (1982) The crystal and melt structures of anorthite at high temperature. J Jpn Assoc Mineral Petrol Econ Geol, Spec Vol 3:19–26 (in Japanese)
Reiss H, Mayer SW (1961) Theory of the surface tension of molten salts. J Chem Phys 34:2001–2003
Reiss H, Frisch HL, Helfand E, Lebowitz JL (1960) Aspects of the statistical thermodynamics of real fluids. J Chem Phys 32:119–124
Richet P (1984) Viscosity and configurational entropy of silicate melts. Geochim Cosmochim Acta 48:471–483
Richet P, Bottinga Y (1984) Anorthite, andesine, wollastonite, cordierite and pyprope: thermodynamics of melting, glass transitions, and properties of the amorphous phases. Earth Planet Sci Lett 67:415–432
Rigden SM, Ahrens TJ, Stolper EM (1984) Densities of liquid silicates at high pressures. Science 226:1071–1074
Ridgen SM, Ahrens TJ, Stolper EM (1988) Shock compression of molten silicate: results for a model basaltic composition. J Geophys Res 93:367–382
Rivers ML, Carmichael ISE (1987) Ultrasonic studies of silicate melts. J Geophys Res 92:9247–9270
Sakka S, Mackenzie JD (1971) Relation between apparent glass transition temperature and liquids temperature for inorganic glass. J Non-Cryst Solids 6:145–162
Scarfe CM, Mysen BO, Virgo D (1979) Changes in viscosity and density of melts of sodium disilicate, sodium metasilicate, and diopside composition with pressure. Carnegie Inst Washington Yearb 78:547–551
Scarfe CM, Cronin DJ, Wenzel JT, Kauffman SA (1983) Viscositytemperature relationships at 1 atm in the system diopside-anorthite. Am Mineral 68:1083–1088
Seifert F, Mysen BO, Virgo D (1982) Three-dimensional network structure of quenched melts (glass) in the system SiO2-NaAlO2, SiO2-CaAl2O4 and SiO2-MgAl2O4. Am Mineral 67:696–717
Shante VKS, Kirkpatrick S (1971) An introduction to percolation theory. Adv Phys 20:325–357
Sharma SK, Simons B, Yoder HS Jr (1983) Raman study of anorthite, calcium Tschermak's pyroxene, and gehlenite in crystalline and glassy states. Am Mineral 68:1113–1125
Shimizu N, Kushiro I (1984) Diffusivity of oxygen in Jadeite and diopside melts at high pressures. Geochim Cosmochim Acta 48:1295–1303
Shiraishi Y, Ikeda K, Tamura A, Sairo T (1978) On the viscosity and density of the molten FeO-SiO2 system. Trans Jpn Inst Met 19:264–274
Stein DJ, Stebbins JF, Carmichael ISE (1986) Density of molten aluminosilicates. J Am Ceram Soc 69:396–399
Stolper E, Walker D, Hager DH, Hays JF (1981) Melt segregation from partially molten source regions: The importance of melt density and source region size. J Geophys Res 86:6261–6271
Sugai M, Somiya S (1982) Measurement of density, viscosity and surface tension of the melt of the system SiO2-TiO2-Al2O3 at 1600° C. Yogyo Kyokai Shi 90:262–269 (in Japanese)
Taniguchi H (1988) Surface tension of melts in the system CaMgSi2O6-CaAl2Si2O8 and its structural significance. Contrib Mineral Petrol 100:484–489
Taniguchi H, Murase T (1986) Physical properties and structures in the melt system diopside-anorthite. Preliminary Rep Spec Res Project 1986:31–34 (in Japanese)
Taniguchi H, Murase T (1987a) Role of free volume in the viscous behavior of magma. J Jpn Assoc Mineral Petrol Econ Geol 82:189–202
Taniguchi H, Murase T (1987b) Some physical properties and melt structures in the system diopside-anorthite. J Volcanol Geotherm Res 34:51–64
Tauber P, Arndt J (1986) Viscosity-temperature relationship of liquid diopside. Phys Earth Planet Int 43:97–103
Taylor M, Brown GE Jr (1979) Structure of mineral glasses-I. The feldspar glasses NaAlSi3O8, KAlSi3O8, CaAl2Si2O8. Geochim Cosmochim Acta 43:61–75
Urbain G, Bottinga Y, Ricbet P (1982) Viscosity of liquid silica, silicates and alumino-silicates. Geochim Cosmochim Acta 46:1061–1072
Weill DF, Hon R, Navrotsky A (1980) The igneous system CaMgSi2O6-CaAl2Si2O8-NaAlSi3O8: variation on a classic theme by Bowen. In: Hargraves RB (ed) Physics of magmatic processes. Princeton University Press, Princeton New Jersey, pp 49–92
Yoder HS Jr (1976) Generation of basaltic magma. National Academy of Sciences, Washington, pp 91
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Taniguchi, H. Densities of melts in the system CaMgSi2O6-CaAl2Si2O8 at low and high pressures, and their structural significance. Contr. Mineral. and Petrol. 103, 325–334 (1989). https://doi.org/10.1007/BF00402919
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00402919