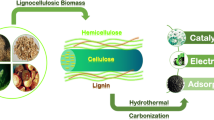
Abstract
Biomass for activated carbon production has had been gaining interest in a wide variety of applications such as water filtration, gas adsorption, and electrochemical devices as a renewable carbon source while meeting desired porosity, surface area, conductivity, and stability requirements. Activated carbon production has been extensively investigated, proving to provide high performance in applications including electrochemical devices. Hydrothermal carbonization (HTC) has shown potential as a pretreatment method for activated carbon production, especially when surface functionalization is desired. However, research into catalytic HTC is still limited. In this review, the processing methods used to convert biomass waste products into high value activated carbon are briefly reviewed, with a focus on recent progress in catalytic HTC as a pretreatment method to activated carbon. Areas of interest for catalytic HTC for activated carbon production are identified. Recent studies have found that the use of catalysts enhances the degree of carbonization, surface modification, and introduction of key heteroatoms significantly augmenting the performance of activated carbon. With further development of catalytic HTC technology, more competent carbon material for electrochemical devices can be produced cost-effectively and move towards meeting the ever-increasing demands of activated carbons for high-performance electrochemical devices.
Graphic Abstract





Similar content being viewed by others
References
Deng, X., Zhao, B., Zhu, L., Shao, Z.: Molten salt synthesis of nitrogen-doped carbon with hierarchical pore structures for use as high-performance electrodes in supercapacitors. Carbon N. Y. 93, 48–58 (2015). https://doi.org/10.1016/j.carbon.2015.05.031
Jin, Y., Tian, K., Wei, L., Zhang, X., Guo, X.: Hierarchical porous microspheres of activated carbon with a high surface area from spores for electrochemical double-layer capacitors. J. Mater. Chem. A. 4, 15968–15979 (2016). https://doi.org/10.1039/c6ta05872h
Liu, Y., Huang, B., Lin, X., Xie, Z.: Biomass-derived hierarchical porous carbons: boosting the energy density of supercapacitors: via an ionothermal approach. J. Mater. Chem. A. 5, 13009–13018 (2017). https://doi.org/10.1039/c7ta03639f
Balakumar, K., Sathish, R., Kalaiselvi, N.: Exploration of microporous bio-carbon scaffold for efficient utilization of sulfur in lithium-sulfur system. Electrochim. Acta. 209, 171–182 (2016). https://doi.org/10.1016/j.electacta.2016.05.069
Liu, Y., Guo, Y., Zhu, Y., An, D., Gao, W., Wang, Z., Ma, Y., Wang, Z.: A sustainable route for the preparation of activated carbon and silica from rice husk ash. J. Hazard. Mater. 186, 1314–1319 (2011). https://doi.org/10.1016/j.jhazmat.2010.12.007
Singh, G., Lakhi, K.S., Kim, I.Y., Kim, S., Srivastava, P., Naidu, R., Vinu, A.: Highly efficient method for the synthesis of activated mesoporous biocarbons with extremely high surface area for high-pressure CO2 adsorption. ACS Appl. Mater. Interfaces. 9, 29782–29793 (2017). https://doi.org/10.1021/acsami.7b08797
Yahya, M.A., Al-Qodah, Z., Ngah, C.W.Z.: Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: a review. Renew. Sustain. Energy Rev. (2015). https://doi.org/10.1016/j.rser.2015.02.051
Deng, J., Li, M., Wang, Y.: Biomass-derived carbon: synthesis and applications in energy storage and conversion. Green Chem. 18, 4824–4854 (2016). https://doi.org/10.1039/c6gc01172a
Abioye, A.M., Ani, F.N.: Recent development in the production of activated carbon electrodes from agricultural waste biomass for supercapacitors: a review. Renew. Sustain. Energy Rev. 52, 1282–1293 (2015). https://doi.org/10.1016/j.rser.2015.07.129
Laine, J., Calafat, A.: Factors affecting the preparation of activated carbons from coconut shell catalized by potassium. Carbon N. Y. 29, 949–953 (1991). https://doi.org/10.1016/0008-6223(91)90173-G
Yang, K., Peng, J., Srinivasakannan, C., Zhang, L., Xia, H., Duan, X.: Preparation of high surface area activated carbon from coconut shells using microwave heating. Bioresour. Technol. 101, 6163–6169 (2010). https://doi.org/10.1016/j.biortech.2010.03.001
Li, W., Yang, K., Peng, J., Zhang, L., Guo, S., Xia, H.: Effects of carbonization temperatures on characteristics of porosity in coconut shell chars and activated carbons derived from carbonized coconut shell chars. Ind. Crops Prod. 28, 190–198 (2008). https://doi.org/10.1016/j.indcrop.2008.02.012
Azevedo, D.C.S., Araújo, J.C.S., Bastos-Neto, M., Torres, A.E.B., Jaguaribe, E.F., Cavalcante, C.L.: Microporous activated carbon prepared from coconut shells using chemical activation with zinc chloride. Microporous Mesoporous Mater. 100, 361–364 (2007). https://doi.org/10.1016/j.micromeso.2006.11.024
Jain, A., Jayaraman, S., Balasubramanian, R., Srinivasan, M.P.: Hydrothermal pre-treatment for mesoporous carbon synthesis: enhancement of chemical activation. J. Mater. Chem. A. 2, 520–528 (2014). https://doi.org/10.1039/C3TA12648J
Sun, L., Tian, C., Li, M., Meng, X., Wang, L., Wang, R., Yin, J., Fu, H.: From coconut shell to porous graphene-like nanosheets for high-power supercapacitors. J. Mater. Chem. A. 1, 6462–6470 (2013). https://doi.org/10.1039/c3ta10897j
Madhu, R., Sankar, K.V., Chen, S.-M., Selvan, R.K.: Eco-friendly synthesis of activated carbon from dead mango leaves for the ultrahigh sensitive detection of toxic heavy metal ions and energy storage applications. RSC Adv. 4, 1225–1233 (2014). https://doi.org/10.1039/C3RA45089A
Gao, S., Fan, H., Zhang, S.: Nitrogen-enriched carbon from bamboo fungus with superior oxygen reduction reaction activity. J. Mater. Chem. A. 2, 18263–18270 (2014). https://doi.org/10.1039/C4TA03558E
Sudaryanto, Y., Hartono, S.B., Irawaty, W., Hindarso, H., Ismadji, S.: High surface area activated carbon prepared from cassava peel by chemical activation. Bioresour. Technol. 97, 734–739 (2006). https://doi.org/10.1016/j.biortech.2005.04.029
Foo, K.Y., Hameed, B.H.: Potential of jackfruit peel as precursor for activated carbon prepared by microwave induced NaOH activation. Bioresour. Technol. 112, 143–150 (2012). https://doi.org/10.1016/j.biortech.2012.01.178
Şentorun-Shalaby, Ç, Uçak-Astarlioǧlu, M.G., Artok, L., Sarici, Ç: Preparation and characterization of activated carbons by one-step steam pyrolysis/activation from apricot stones. Microporous Mesoporous Mater. 88, 126–134 (2006). https://doi.org/10.1016/j.micromeso.2005.09.003
Martínez, M.L., Torres, M.M., Guzmán, C.A., Maestri, D.M.: Preparation and characteristics of activated carbon from olive stones and walnut shells. Ind. Crops Prod. 23, 23–28 (2006). https://doi.org/10.1016/j.indcrop.2005.03.001
Caturla, F., Molina-Sabio, M., Rodríguez-Reinoso, F.: Preparation of activated carbon by chemical activation with ZnCl2. Carbon N. Y. 29, 999–1007 (1991). https://doi.org/10.1016/0008-6223(91)90179-M
Dolas, H., Sahin, O., Saka, C., Demir, H.: A new method on producing high surface area activated carbon: the effect of salt on the surface area and the pore size distribution of activated carbon prepared from pistachio shell. Chem. Eng. J. 166, 191–197 (2011). https://doi.org/10.1016/j.cej.2010.10.061
Aygün, A., Yenisoy-Karakaş, S., Duman, I.: Production of granular activated carbon from fruit stones and nutshells and evaluation of their physical, chemical and adsorption properties. Microporous Mesoporous Mater. 66, 189–195 (2003). https://doi.org/10.1016/j.micromeso.2003.08.028
Ahmadpour, A., Do, D.D.: The preparation of activated carbon from macadamia nutshell by chemical activation. Carbon N. Y. 35, 1723–1732 (1997). https://doi.org/10.1016/S0008-6223(97)00127-9
Girgis, B.S., Yunis, S.S., Soliman, A.M.: Characteristics of activated carbon from peanut hulls in relation to conditions of preparation. Mater. Lett. 57, 164–172 (2002). https://doi.org/10.1016/S0167-577X(02)00724-3
Evans, M.J.B., Halliop, E., MacDonald, J.A.F.: The production of chemically-activated carbon. Carbon N. Y. 37, 269–274 (1999). https://doi.org/10.1016/S0008-6223(98)00174-2
Oliveira, L.C.A., Pereira, E., Guimaraes, I.R., Vallone, A., Pereira, M., Mesquita, J.P., Sapag, K.: Preparation of activated carbons from coffee husks utilizing FeCl3 and ZnCl2 as activating agents. J. Hazard. Mater. 165, 87–94 (2009). https://doi.org/10.1016/j.jhazmat.2008.09.064
Zhang, T., Walawender, W.P., Fan, L.T., Fan, M., Daugaard, D., Brown, R.C.: Preparation of activated carbon from forest and agricultural residues through CO2 activation. Chem. Eng. J. 105, 53–59 (2004). https://doi.org/10.1016/j.cej.2004.06.011
Cagnon, B., Py, X., Guillot, A., Stoeckli, F., Chambat, G.: Contributions of hemicellulose, cellulose and lignin to the mass and the porous properties of chars and steam activated carbons from various lignocellulosic precursors. Bioresour. Technol. 100, 292–298 (2009). https://doi.org/10.1016/j.biortech.2008.06.009
Foo, K.Y., Hameed, B.H.: Mesoporous activated carbon from wood sawdust by K2CO3activation using microwave heating. Bioresour. Technol. 111, 425–432 (2012). https://doi.org/10.1016/j.biortech.2012.01.141
Khezami, L., Ould-Dris, A., Capart, R.: Activated carbon from thermo-compressed wood and other lignocellulosic precursors. BioResources. 2, 193–209 (2007). https://doi.org/10.15376/biores.2.2.193-209
Nabais, J.M.V., Laginhas, C., Carrott, M.M.L.R., Carrott, P.J.M., Amorós, J.E.C., Gisbert, A.V.N.: Surface and porous characterisation of activated carbons made from a novel biomass precursor, the esparto grass. Appl. Surf. Sci. 265, 919–924 (2013). https://doi.org/10.1016/j.apsusc.2012.11.164
Falco, C., Marco-Lozar, J.P., Salinas-Torres, D., Morallón, E., Cazorla-Amorós, D., Titirici, M.M., Lozano-Castelló, D.: Tailoring the porosity of chemically activated hydrothermal carbons: influence of the precursor and hydrothermal carbonization temperature. Carbon N. Y. 62, 346–355 (2013). https://doi.org/10.1016/j.carbon.2013.06.017
Kalderis, D., Bethanis, S., Paraskeva, P., Diamadopoulos, E.: Production of activated carbon from bagasse and rice husk by a single-stage chemical activation method at low retention times. Bioresour. Technol. 99, 6809–6816 (2008). https://doi.org/10.1016/j.biortech.2008.01.041
Kaghazchi, T., Kolur, N.A., Soleimani, M.: Licorice residue and pistachio-nut shell mixture: a promising precursor for activated carbon. J. Ind. Eng. Chem. 16, 368–374 (2010). https://doi.org/10.1016/j.jiec.2009.10.002
Heschel, W., Klose, E.: On the suitability of agricultural by-products for the manufacture of granular activated carbon. Fuel. 74, 1786–1791 (1995). https://doi.org/10.1016/0016-2361(95)80009-7
Guo, F., Fang, Z.: Shape-controlled synthesis of activated bio-chars by surfactant-templated ionothermal carbonization in acidic ionic liquid and activation with carbon dioxide. BioResources. 9, 3369–3383 (2014). https://doi.org/10.15376/biores.9.2.3369-3383
Yagmur, E., Ozmak, M., Aktas, Z.: A novel method for production of activated carbon from waste tea by chemical activation with microwave energy. Fuel. 87, 3278–3285 (2008). https://doi.org/10.1016/j.fuel.2008.05.005
Suzuki, R.M., Andrade, A.D., Sousa, J.C., Rollemberg, M.C.: Preparation and characterization of activated carbon from rice bran. Bioresour. Technol. 98, 1985–1991 (2007). https://doi.org/10.1016/j.biortech.2006.08.001
Diao, Y., Walawender, W., Fan, L.: Activated carbons prepared from phosphoric acid activation of grain sorghum. Bioresour. Technol. 81, 45–52 (2002). https://doi.org/10.1016/S0960-8524(01)00100-6
Zhao, L., Fan, L.Z., Zhou, M.Q., Guan, H., Qiao, S., Antonietti, M., Titirici, M.M.: Nitrogen-containing hydrothermal carbons with superior performance in supercapacitors. Adv. Mater. 22, 5202–5206 (2010). https://doi.org/10.1002/adma.201002647
Lozano-Castelló, D., Lillo-Ródenas, M.A., Cazorla-Amorós, D., Linares-Solano, A.: Preparation of activated carbons from Spanish anthracite I. Activation by KOH. Carbon N. Y. 39, 741–749 (2001). https://doi.org/10.1016/S0008-6223(00)00185-8
Lillo-Ródenas, M., Cazorla-Amorós, D., Linares-Solano, A., Rodenas, M., Amoros, D., Solano, A.: Understanding chemical reactions between carbons and NaOH and KOH An insight into the chemical activation mechanism. Carbon N. Y. 41, 267–275 (2003). https://doi.org/10.1016/S0008-6223(02)00279-8
Prahas, D., Kartika, Y., Indraswati, N., Ismadji, S.: Activated carbon from jackfruit peel waste by H3PO4 chemical activation: pore structure and surface chemistry characterization. Chem. Eng. J. 140, 32–42 (2008). https://doi.org/10.1016/j.cej.2007.08.032
Lim, W.C., Srinivasakannan, C., Balasubramanian, N.: Activation of palm shells by phosphoric acid impregnation for high yielding activated carbon. J. Anal. Appl. Pyrolysis. 88, 181–186 (2010). https://doi.org/10.1016/j.jaap.2010.04.004
Tay, T., Ucar, S., Karagöz, S.: Preparation and characterization of activated carbon from waste biomass. J. Hazard. Mater. 165, 481–485 (2009). https://doi.org/10.1016/j.jhazmat.2008.10.011
Tongpoothorn, W., Sriuttha, M., Homchan, P., Chanthai, S., Ruangviriyachai, C.: Preparation of activated carbon derived from Jatropha curcas fruit shell by simple thermo-chemical activation and characterization of their physico-chemical properties. Chem. Eng. Res. Des. 89, 335–340 (2011). https://doi.org/10.1016/j.cherd.2010.06.012
Fu, K., Yue, Q., Gao, B., Sun, Y., Wang, Y., Li, Q., Zhao, P., Chen, S.: Physicochemical and adsorptive properties of activated carbons from Arundo donax Linn utilizing different iron salts as activating agents. J. Taiwan Inst. Chem. Eng. 45, 3007–3015 (2014). https://doi.org/10.1016/j.jtice.2014.08.026
Theydan, S.K., Ahmed, M.J.: Optimization of preparation conditions for activated carbons from date stones using response surface methodology. Powder Technol. 224, 101–108 (2012). https://doi.org/10.1016/j.powtec.2012.02.037
Legrouri, K., Khouya, E., Ezzine, M., Hannache, H., Denoyel, R., Pallier, R., Naslain, R.: Production of activated carbon from a new precursor molasses by activation with sulphuric acid. J. Hazard. Mater. 118, 259–263 (2005). https://doi.org/10.1016/j.jhazmat.2004.11.004
El-Hendawy, A.A.: An insight into KOH activation mechanism via production of microporous activated carbon for heavy metal removal. Egypt. J. Chem. 51, 681–700 (2008). https://doi.org/10.1016/j.apsusc.2008.10.034
Hjaila, K., Baccar, R., Sarrà, M., Gasol, C.M., Blánquez, P.: Environmental impact associated with activated carbon preparation from olive-waste cake via life cycle assessment. J. Environ. Manag. 130, 242–247 (2013). https://doi.org/10.1016/j.jenvman.2013.08.061
Lim, W.C., Srinivasakannan, C., Al Shoaibi, A.: Cleaner production of porous carbon from palm shells through recovery and reuse of phosphoric acid. J. Clean. Prod. 102, 501–511 (2015). https://doi.org/10.1016/j.jclepro.2015.04.100
Sevilla, M., Fuertes, A.B.: A green approach to high-performance supercapacitor electrodes: the chemical activation of hydrochar with potassium bicarbonate. ChemSusChem. 9, 1880–1888 (2016). https://doi.org/10.1002/cssc.201600426
Gong, Y., Wei, Z., Wang, J., Zhang, P., Li, H., Wang, Y.: Design and fabrication of hierarchically porous carbon with a template-free method. Sci. Rep. 4, 1–6 (2014). https://doi.org/10.1038/srep06349
Khalili, N.R., Campbell, M., Sandi, G., Golaś, J.: Production of micro- and mesoporous activated carbon from paper mill sludge. I. Effect of zinc chloride activation. Carbon N. Y. 38, 1905–1915 (2000). https://doi.org/10.1016/S0008-6223(00)00043-9
Bouchelta, C., Medjram, M.S., Bertrand, O., Bellat, J.P.: Preparation and characterization of activated carbon from date stones by physical activation with steam. J. Anal. Appl. Pyrolysis. 82, 70–77 (2008). https://doi.org/10.1016/j.jaap.2007.12.009
Rodríguez-Reinoso, F., Molina-Sabio, M.: Activated carbons from lignocellulosic materials by chemical and/or physical activation: an overview. Carbon N. Y. 30, 1111–1118 (1992). https://doi.org/10.1016/0008-6223(92)90143-K
Xin-Hui, D., Srinivasakannan, C., Jin-Hui, P., Li-Bo, Z., Zheng-Yong, Z.: Preparation of activated carbon from Jatropha hull with microwave heating: optimization using response surface methodology. Fuel Process. Technol. 92, 394–400 (2011). https://doi.org/10.1016/j.fuproc.2010.09.033
Wigmans, T.: Industrial aspects of production and use of activated carbons. Carbon N. Y. 27, 13–22 (1989). https://doi.org/10.1016/0008-6223(89)90152-8
Jain, A., Balasubramanian, R., Srinivasan, M.P.: Hydrothermal conversion of biomass waste to activated carbon with high porosity: a review. Chem. Eng. J. (2016). https://doi.org/10.1016/j.cej.2015.08.014
Gao, Z., Zhang, Y., Song, N., Li, X.: Biomass-derived renewable carbon materials for electrochemical energy storage. Mater. Res. Lett. 5, 69–88 (2017). https://doi.org/10.1080/21663831.2016.1250834
Li, B., Dai, F., Xiao, Q., Yang, L., Shen, J., Zhang, C., Cai, M.: Nitrogen-doped activated carbon for a high energy hybrid supercapacitor. Energy Environ. Sci. 9, 102–106 (2016). https://doi.org/10.1039/c5ee03149d
Occelli, M.L., Olivier, J.P., Peridon-Melon, J.A., Auroux, A.: Surface area, pore volume distribution, and acidity in mesoporous expanded clay catalysts from hybrid density functional theory (DFT) and adsorption microcalorimetry methods. Langmuir. 18, 9816–9823 (2002). https://doi.org/10.1021/la020567o
Barbieri, O., Hahn, M., Herzog, A., Kötz, R.: Capacitance limits of high surface area activated carbons for double layer capacitors. Carbon N. Y. 43, 1303–1310 (2005). https://doi.org/10.1016/j.carbon.2005.01.001
Wang, D.W., Li, F., Liu, M., Lu, G.Q., Cheng, H.M.: 3D aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage. Angew. Chemie - Int. Ed. 47, 373–376 (2008). https://doi.org/10.1002/anie.200702721
Liu, C., Yan, X., Hu, F., Gao, G., Wu, G., Yang, X.: Toward superior capacitive energy storage: recent advances in pore engineering for dense electrodes. Adv. Mater. 30, 1–14 (2018). https://doi.org/10.1002/adma.201705713
Demir, M., Saraswat, S.K., Gupta, R.B.: Hierarchical nitrogen-doped porous carbon derived from lecithin for high-performance supercapacitors. RSC Adv. 7, 42430–42442 (2017). https://doi.org/10.1039/c7ra07984b
Largeot, C., Portet, C., Chmiola, J., Taberna, P.L., Gogotsi, Y., Simon, P.: Relation between the ion size and pore size for an electric double-layer capacitor. J. Am. Chem. Soc. 130, 2730–2731 (2008). https://doi.org/10.1021/ja7106178
Jäckel, N., Rodner, M., Schreiber, A., Jeongwook, J., Zeiger, M., Aslan, M., Weingarth, D., Presser, V.: Anomalous or regular capacitance? The influence of pore size dispersity on double-layer formation. J. Power Sources. 326, 660–671 (2016). https://doi.org/10.1016/j.jpowsour.2016.03.015
Endo, M., Maeda, T., Takeda, T., Kim, Y.J., Koshiba, K., Hara, H., Dresselhaus, M.S.: Capacitance and pore-size distribution in aqueous and nonaqueous electrolytes using various activated carbon electrodes. J. Electrochem. Soc. 148, A910 (2002). https://doi.org/10.1149/1.1382589
Salitra, G., Soffer, A., Eliad, L., Cohen, Y., Aurbach, D.: Carbon electrodes for double-layer capacitors I. Relations between ion and pore dimensions. J. Electrochem. Soc. 147, 2486 (2000). https://doi.org/10.1149/1.1393557
Zuliani, J.E., Tong, S., Kirk, D.W., Jia, C.Q.: Isolating the effect of pore size distribution on electrochemical double-layer capacitance using activated fluid coke. J. Power Sources. 300, 190–198 (2015). https://doi.org/10.1016/j.jpowsour.2015.09.030
Jäckel, N., Simon, P., Gogotsi, Y., Presser, V.: Increase in capacitance by subnanometer pores in carbon. ACS Energy Lett. 1, 1262–1265 (2016). https://doi.org/10.1021/acsenergylett.6b00516
Barczak, M., Elsayed, Y., Jagiello, J., Bandosz, T.J.: Exploring the effect of ultramicropore distribution on gravimetric capacitance of nanoporous carbons. Electrochim. Acta 275, 236–247 (2018). https://doi.org/10.1016/j.electacta.2018.04.035
Redondo, E., Carretero-González, J., Goikolea, E., Ségalini, J., Mysyk, R.: Effect of pore texture on performance of activated carbon supercapacitor electrodes derived from olive pits. Electrochim. Acta 160, 178–184 (2015). https://doi.org/10.1016/j.electacta.2015.02.006
Sevilla, M., Fuertes, A.B.: Catalytic graphitization of templated mesoporous carbons. Carbon N. Y. 44, 468–474 (2006). https://doi.org/10.1016/j.carbon.2005.08.019
Hsieh, C.T., Teng, H.: Influence of oxygen treatment on electric double-layer capacitance of activated carbon fabrics. Carbon N. Y. 40, 667–674 (2002). https://doi.org/10.1016/S0008-6223(01)00182-8
Lota, G., Grzyb, B., Machnikowska, H., Machnikowski, J., Frackowiak, E.: Effect of nitrogen in carbon electrode on the supercapacitor performance. Chem. Phys. Lett. 404, 53–58 (2005). https://doi.org/10.1016/j.cplett.2005.01.074
Kiciński, W., Szala, M., Bystrzejewski, M.: Sulfur-doped porous carbons: synthesis and applications. Carbon N. Y. 68, 1–32 (2014). https://doi.org/10.1016/j.carbon.2013.11.004
Qu, D.: Studies of the activated carbons used in double-layer supercapacitors. J. Power Sour. 109, 403–411 (2002). https://doi.org/10.1016/S0378-7753(02)00108-8
Jung, A., Han, S., Kim, T., Cho, W.J., Lee, K.H.: Synthesis of high carbon content microspheres using 2-step microwave carbonization, and the influence of nitrogen doping on catalytic activity. Carbon N. Y. 60, 307–316 (2013). https://doi.org/10.1016/j.carbon.2013.04.042
Hu, C., Dai, L.: Doping of carbon materials for metal-free electrocatalysis. Adv. Mater. 1804672, 1804672 (2018). https://doi.org/10.1002/adma.201804672
Hoekman, S.K., Broch, A., Robbins, C.: Hydrothermal carbonization (HTC) of lignocellulosic biomass. Energy & Fuels. 25, 1802–1810 (2011). https://doi.org/10.1021/ef101745n
Kumabe, K., Itoh, N., Matsumoto, K., Hasegawa, T.: Hydrothermal gasification of glucose and starch in a batch and continuous reactor. Energy Rep. 3, 70–75 (2017). https://doi.org/10.1016/j.egyr.2017.04.001
Zhao, Y., Singh, A.K., Jang, S., Wang, A., Kim, D.P.: Continuous-flow synthesis of functional carbonaceous particles from biomass under hydrothermal carbonization. J. Flow Chem. 4, 195–199 (2014). https://doi.org/10.1556/JFC-D-14-00018
Hoekman, S.K., Broch, A., Felix, L., Farthing, W.: Hydrothermal carbonization (HTC) of loblolly pine using a continuous, reactive twin-screw extruder. Energy Convers. Manag. 134, 247–259 (2017). https://doi.org/10.1016/j.enconman.2016.12.035
Hoekman, S.K., Broch, A., Robbins, C., Purcell, R., Zielinska, B., Felix, L., Irvin, J.: Process Development Unit (PDU) for hydrothermal carbonization (HTC) of lignocellulosic biomass. Waste and Biomass Valorization. 5, 669–678 (2014). https://doi.org/10.1007/s12649-013-9277-0
Köchermann, J., Görsch, K., Wirth, B., Mühlenberg, J., Klemm, M.: Hydrothermal carbonization: temperature influence on hydrochar and aqueous phase composition during process water recirculation. J. Environ. Chem. Eng. 6, 5481–5487 (2018). https://doi.org/10.1016/j.jece.2018.07.053
Kabadayi Catalkopru, A., Kantarli, I.C., Yanik, J.: Effects of spent liquor recirculation in hydrothermal carbonization. Bioresour. Technol. 226, 89–93 (2017). https://doi.org/10.1016/j.biortech.2016.12.015
Reza, M.T., Lynam, J.G., Uddin, M.H., Coronella, C.J.: Hydrothermal carbonization: fate of inorganics. Biomass and Bioenergy. 49, 86–94 (2013). https://doi.org/10.1016/j.biombioe.2012.12.004
Funke, A., Ziegler, F.: Hydrothermal carbonization of biomass: a summary and discussion of chemical mechanisms for process engineering. Biofuels, Bioprod. Biorefining. 4, 160–177 (2010). https://doi.org/10.1002/bbb.198
Heidari, M., Dutta, A., Acharya, B., Mahmud, S.: A review of the current knowledge and challenges of hydrothermal carbonization for biomass conversion. J. Energy Inst. (2018). https://doi.org/10.1016/j.joei.2018.12.003
Khan, T.A., Saud, A.S., Jamari, S.S., Rahim, M.H.A., Park, J.W., Kim, H.J.: Hydrothermal carbonization of lignocellulosic biomass for carbon rich material preparation: a review. Biomass and Bioenergy. 130, 105384 (2019). https://doi.org/10.1016/j.biombioe.2019.105384
Barskov, S., Zappi, M., Buchireddy, P., Dufreche, S., Guillory, J., Gang, D., Hernandez, R., Bajpai, R., Baudier, J., Cooper, R., Sharp, R.: Torrefaction of biomass: a review of production methods for biocoal from cultured and waste lignocellulosic feedstocks. Renew. Energy. 142, 624–642 (2019). https://doi.org/10.1016/j.renene.2019.04.068
Rodriguez Correa, C., Kruse, A.: Supercritical water gasification of biomass for hydrogen production—review. J. Supercrit. Fluids 133, 573–590 (2018). https://doi.org/10.1016/j.supflu.2017.09.019
Luo, L., Chen, T., Zhao, W., Fan, M.: Hydrothermal doping of nitrogen in bamboo-based super activated carbon for hydrogen storage. BioResources. 12, 6237–6250 (2017). https://doi.org/10.15376/biores.12.3.6237-6250
Luo, L., Xu, C., Chen, Z., Zhang, S.: Properties of biomass-derived biochars: combined effects of operating conditions and biomass types. Bioresour. Technol. 192, 83–89 (2015). https://doi.org/10.1016/j.biortech.2015.05.054
Lee, J.S., Mayes, R.T., Luo, H., Dai, S.: Ionothermal carbonization of sugars in a protic ionic liquid under ambient conditions. Carbon N. Y. 48, 3364–3368 (2010). https://doi.org/10.1016/j.carbon.2010.05.027
Hossain, M.M.: Promotional effects of Ce on Ni–Ce/ΓAl2O3 for enhancement of H2 in hydrothermal gasification of biomass. Int. J. Hydrogen Energy 43, 6088–6095 (2018). https://doi.org/10.1016/j.ijhydene.2018.01.182
Jiao, J.L., Wang, F., Duan, P.G., Xu, Y.P., Yan, W.H.: Catalytic hydrothermal gasification of microalgae for producing hydrogen and methane-rich gas. Energy Sour. Part A Recover Util. Environ. Eff. 39, 851–860 (2017). https://doi.org/10.1080/15567036.2016.1270375
Sert, M., Selvi Gökkaya, D., Cengiz, N., Ballice, L., Yüksel, M., Sağlam, M.: Hydrogen production from olive-pomace by catalytic hydrothermal gasification. J. Taiwan Inst. Chem. Eng. 83, 90–98 (2018). https://doi.org/10.1016/j.jtice.2017.11.026
Watson, J., Si, B., Li, H., Liu, Z., Zhang, Y.: Influence of catalysts on hydrogen production from wastewater generated from the HTL of human feces via catalytic hydrothermal gasification. Int. J. Hydrogen Energy 42, 20503–20511 (2017). https://doi.org/10.1016/j.ijhydene.2017.05.083
Chen, J., Wang, L., Zhang, B., Li, R., Shahbazi, A.: Hydrothermal liquefaction enhanced by various chemicals as a means of sustainable dairy manure treatment. Sustainability. 10, 230 (2018). https://doi.org/10.3390/su10010230
Lu, X., Flora, J.R.V., Berge, N.D.: Influence of process water quality on hydrothermal carbonization of cellulose. Bioresour. Technol. 154, 229–239 (2014). https://doi.org/10.1016/j.biortech.2013.11.069
Reza, M.T., Rottler, E., Herklotz, L., Wirth, B.: Hydrothermal carbonization (HTC) of wheat straw: influence of feedwater pH prepared by acetic acid and potassium hydroxide. Bioresour. Technol. 182, 336–344 (2015). https://doi.org/10.1016/j.biortech.2015.02.024
Lu, Y., Mosier, N.S.: Kinetic modeling analysis of maleic acid-catalyzed hemicellulose hydrolysis in corn stover. Biotechnol. Bioeng. 101, 1170–1181 (2008). https://doi.org/10.1002/bit.22008
Wang, T., Zhai, Y., Zhu, Y., Li, C., Zeng, G.: A review of the hydrothermal carbonization of biomass waste for hydrochar formation: process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 90, 223–247 (2018). https://doi.org/10.1016/j.rser.2018.03.071
Dapsens, P.Y., Mondelli, C., Pérez-Ramírez, J.: Biobased chemicals from conception toward industrial reality: lessons learned and to be learned. ACS Catal. 2, 1487–1499 (2012). https://doi.org/10.1021/cs300124m
Krylova, A.Y., Zaitchenko, V.M.: Hydrothermal carbonization of biomass: a review. Solid Fuel Chem. 52, 91–103 (2018). https://doi.org/10.3103/S0361521918020076
Kumar, M., Olajire Oyedun, A., Kumar, A.: A review on the current status of various hydrothermal technologies on biomass feedstock. Renew. Sustain. Energy Rev. 81, 1742–1770 (2018). https://doi.org/10.1016/j.rser.2017.05.270
Titirici, M.M., White, R.J., Falco, C., Sevilla, M.: Black perspectives for a green future: hydrothermal carbons for environment protection and energy storage. Energy Environ. Sci. 5, 6796–6822 (2012). https://doi.org/10.1039/c2ee21166a
Susanti, R.F., Arie, A.A., Kristianto, H., Erico, M., Kevin, G., Devianto, H.: Activated carbon from citric acid catalyzed hydrothermal carbonization and chemical activation of salacca peel as potential electrode for lithium ion capacitor’s cathode. Ionics. 25, 3915–3925 (2019). https://doi.org/10.1007/s11581-019-02904-x
Gan, L., Zhu, J., Lv, L.: Cellulose hydrolysis catalyzed by highly acidic lignin-derived carbonaceous catalyst synthesized via hydrothermal carbonization. Cellulose. 24, 5327–5339 (2017). https://doi.org/10.1007/s10570-017-1515-3
Zhou, N., Chen, H., Feng, Q., Yao, D., Chen, H., Wang, H., Zhou, Z., Li, H., Tian, Y., Lu, X.: Effect of phosphoric acid on the surface properties and Pb(II) adsorption mechanisms of hydrochars prepared from fresh banana peels. J. Clean. Prod. 165, 221–230 (2017). https://doi.org/10.1016/j.jclepro.2017.07.111
Zhao, Q., Tao, S., Miao, X., Zhu, Y.: A green, rapid, scalable and versatile hydrothermal strategy to fabricate monodisperse carbon spheres with tunable micrometer size and hierarchical porosity. Chem. Eng. J. 372, 1164–1173 (2019). https://doi.org/10.1016/j.cej.2019.05.014
Fechler, N., Wohlgemuth, S.A., Jäker, P., Antonietti, M.: Salt and sugar: direct synthesis of high surface area carbon materials at low temperatures via hydrothermal carbonization of glucose under hypersaline conditions. J. Mater. Chem. A. 1, 9418–9421 (2013). https://doi.org/10.1039/c3ta10674h
Lynam, J.G., Coronella, C.J., Yan, W., Reza, M.T., Vasquez, V.R.: Acetic acid and lithium chloride effects on hydrothermal carbonization of lignocellulosic biomass. Bioresour. Technol. 102, 6192–6199 (2011). https://doi.org/10.1016/j.biortech.2011.02.035
Hamid, S.B.A., Teh, S.J., Lim, Y.S.: Catalytic hydrothermal upgrading of α-cellulose using iron salts as a lewis acid. BioResources. 10, 5974–5986 (2015). https://doi.org/10.15376/biores.10.3.5974-5986
Liu, X., Antonietti, M.: Molten salt activation for synthesis of porous carbon nanostructures and carbon sheets. Carbon N. Y. 69, 460–466 (2014). https://doi.org/10.1016/j.carbon.2013.12.049
Marsh, H., Crawford, D., Taylor, D.W.: Catalytic graphitization by iron of isotropic carbon from polyfurfuryl alcohol. Carbon N. Y. 21, 81–87 (1983). https://doi.org/10.1016/0008-6223(83)90160-4
Yudasaka, M., Tasaka, K., Kikuchi, R., Ohki, Y., Yoshimura, S., Ota, E.: Influence of chemical bond of carbon on Ni catalyzed graphitization. J. Appl. Phys. 81, 7623–7629 (1997). https://doi.org/10.1063/1.365339
Mochida, I., Ohtsubo, R., Takeshita, K., Marsh, H.: Catalytic graphitization of non-graphitizable carbon by chromium and manganese oxides. Carbon N. Y. 18, 117–123 (1980). https://doi.org/10.1016/0008-6223(80)90019-6
García-Bordejé, E., Pires, E., Fraile, J.M.: Parametric study of the hydrothermal carbonization of cellulose and effect of acidic conditions. Carbon N. Y. 123, 421–432 (2017). https://doi.org/10.1016/j.carbon.2017.07.085
Simsir, H., Eltugral, N., Karagoz, S.: Effects of acidic and alkaline metal triflates on the hydrothermal carbonization of glucose and cellulose. Energy & Fuels. 33, 7473–7479 (2019). https://doi.org/10.1021/acs.energyfuels.9b01750
Rather, M.A., Khan, N.S., Gupta, R.: Catalytic hydrothermal carbonization of invasive macrophyte Hornwort (Ceratophyllum demersum) for production of hydrochar: a potential biofuel. Int. J. Environ. Sci. Technol. 14, 1243–1252 (2017). https://doi.org/10.1007/s13762-016-1227-5
Lee, K.U., Kim, M.J., Park, K.J., Kim, M., Kwon, O.J., Kim, J.J.: Catalytic growth of a colloidal carbon sphere by hydrothermal reaction with iron oxide (Fe3O4) catalyst. Mater. Lett. 125, 213–217 (2014). https://doi.org/10.1016/j.matlet.2014.03.163
Gu, L., Li, B., Wen, H., Zhang, X., Wang, L., Ye, J.: Co-hydrothermal treatment of fallen leaves with iron sludge to prepare magnetic iron product and solid fuel. Bioresour. Technol. 257, 229–237 (2018). https://doi.org/10.1016/j.biortech.2018.02.113
Han, C., Wang, S., Wang, J., Li, M., Deng, J., Li, H., Wang, Y.: Controlled synthesis of sustainable N-doped hollow core-mesoporous shell carbonaceous nanospheres from biomass. Nano Res. 7, 1809–1819 (2014). https://doi.org/10.1007/s12274-014-0540-x
Kubo, S., White, R.J., Yoshizawa, N., Antonietti, M., Titirici, M.M.: Ordered carbohydrate-derived porous carbons. Chem. Mater. 23, 4882–4885 (2011). https://doi.org/10.1021/cm2020077
Kubo, S., Demir-Cakan, R., Zhao, L., White, R.J., Titirici, M.M.: Porous carbohydrate-based materials via hard templating. ChemSusChem. 3, 188–194 (2010). https://doi.org/10.1002/cssc.200900126
Krishnan, D., Raidongia, K., Shao, J., Huang, J.: Graphene oxide assisted hydrothermal carbonization of carbon hydrates. ACS Nano. 8, 449–457 (2014). https://doi.org/10.1021/nn404805p
Hu, Y., Liu, H., Ke, Q., Wang, J.: Effects of nitrogen doping on supercapacitor performance of a mesoporous carbon electrode produced by a hydrothermal soft-templating process. J. Mater. Chem. A. 2, 11753–11758 (2014). https://doi.org/10.1039/c4ta01269k
Xiao, P.W., Zhao, L., Sui, Z.Y., Xu, M.Y., Han, B.H.: Direct synthesis of ordered mesoporous hydrothermal carbon materials via a modified soft-templating method. Microporous Mesoporous Mater. 253, 215–222 (2017). https://doi.org/10.1016/j.micromeso.2017.07.001
Xiao, P.W., Guo, D., Zhao, L., Han, B.H.: Soft templating synthesis of nitrogen-doped porous hydrothermal carbons and their applications in carbon dioxide and hydrogen adsorption. Microporous Mesoporous Mater. 220, 129–135 (2016). https://doi.org/10.1016/j.micromeso.2015.08.027
Song, L.T., Wu, Z.Y., Liang, H.W., Zhou, F., Yu, Z.Y., Xu, L., Pan, Z., Yu, S.H.: Macroscopic-scale synthesis of nitrogen-doped carbon nanofiber aerogels by template-directed hydrothermal carbonization of nitrogen-containing carbohydrates. Nano Energy 19, 117–127 (2016). https://doi.org/10.1016/j.nanoen.2015.10.004
Wu, Q., Gao, M., Zhang, G., Zhang, Y., Liu, S., Xie, C., Yu, H., Liu, Y., Huang, L., Yu, S.: Preparation and application performance study of biomass-based carbon materials with various morphologies by a hydrothermal/soft template method. Nanotechnology. 30, 18572 (2019). https://doi.org/10.1088/1361-6528/ab0042
Kruse, A., Koch, F., Stelzl, K., Wüst, D., Zeller, M.: Fate of nitrogen during hydrothermal carbonization. Energy Fuels. 30, 8037–8042 (2016). https://doi.org/10.1021/acs.energyfuels.6b01312
Wang, T., Zhai, Y., Zhu, Y., Wang, Z., Xiao, H., Peng, C., Wang, B., Li, C.: What is the influence of the nitrogen-containing composition during hydrothermal carbonization of biomass? A new perspective from mimic feedstock. Bioresour. Technol. Rep. 5, 343–350 (2019). https://doi.org/10.1016/j.biteb.2018.07.001
Sevilla, M., Gu, W., Falco, C., Titirici, M.M., Fuertes, A.B., Yushin, G.: Hydrothermal synthesis of microalgae-derived microporous carbons for electrochemical capacitors. J. Power Sour. 267, 26–32 (2014). https://doi.org/10.1016/j.jpowsour.2014.05.046
Gai, C., Guo, Y., Peng, N., Liu, T., Liu, Z.: N-Doped biochar derived from co-hydrothermal carbonization of rice husk and: Chlorella pyrenoidosa for enhancing copper ion adsorption. RSC Adv. 6, 53713–53722 (2016). https://doi.org/10.1039/c6ra09270e
Hou, L., Hu, Z., Wang, X., Qiang, L., Zhou, Y., Lv, L., Li, S.: Hierarchically porous and heteroatom self-doped graphitic biomass carbon for supercapacitors. J. Colloid Interface Sci. 540, 88–96 (2019). https://doi.org/10.1016/j.jcis.2018.12.029
Deng, P., Lei, S., Wang, W., Zhou, W., Ou, X., Chen, L., Xiao, Y., Cheng, B.: Conversion of biomass waste to multi-heteroatom-doped carbon networks with high surface area and hierarchical porosity for advanced supercapacitors. J. Mater. Sci. 53, 14536–14547 (2018). https://doi.org/10.1007/s10853-018-2630-8
Sun, Y., Liu, C., Zan, Y., Miao, G., Wang, H., Kong, L.: Hydrothermal carbonization of microalgae (Chlorococcum sp.) for porous carbons with high Cr(VI) adsorption performance. Appl. Biochem. Biotechnol. 186, 414–424 (2018). https://doi.org/10.1007/s12010-018-2752-0
Liu, H., Chen, Y., Yang, H., Gentili, F.G., Söderlind, U., Wang, X., Zhang, W., Chen, H.: Hydrothermal carbonization of natural microalgae containing a high ash content. Fuel. 249, 441–448 (2019). https://doi.org/10.1016/j.fuel.2019.03.004
Jain, A., Balasubramanian, R., Srinivasan, M.P.: Production of high surface area mesoporous activated carbons from waste biomass using hydrogen peroxide-mediated hydrothermal treatment for adsorption applications. Chem. Eng. J. 273, 622–629 (2015). https://doi.org/10.1016/j.cej.2015.03.111
Schipper, F., Kubo, S., Fellinger, T.P.: Nitrogen-doped porous carbon via ammonothermal carbonization for supercapacitors. J. Sol–Gel. Sci. Technol. 89, 101–110 (2019). https://doi.org/10.1007/s10971-018-4837-1
Ghanim, B.M., Kwapinski, W., Leahy, J.J.: Hydrothermal carbonisation of poultry litter: effects of initial pH on yields and chemical properties of hydrochars. Bioresour. Technol. 238, 78–85 (2017). https://doi.org/10.1016/j.biortech.2017.04.025
Liu, S., Cai, Y., Zhao, X., Liang, Y., Zheng, M., Hu, H., Dong, H., Jiang, S., Liu, Y., Xiao, Y.: Sulfur-doped nanoporous carbon spheres with ultrahigh specific surface area and high electrochemical activity for supercapacitor. J. Power Sour. 360, 373–382 (2017). https://doi.org/10.1016/j.jpowsour.2017.06.029
Choi, C.H., Chung, M.W., Park, S.H., Woo, S.I.: Additional doping of phosphorus and/or sulfur into nitrogen-doped carbon for efficient oxygen reduction reaction in acidic media. Phys. Chem. Chem. Phys. 15, 1802–1805 (2013). https://doi.org/10.1039/c2cp44147k
Si, W., Zhou, J., Zhang, S., Li, S., Xing, W., Zhuo, S.: Tunable N-doped or dual N, S-doped activated hydrothermal carbons derived from human hair and glucose for supercapacitor applications. Electrochim. Acta 107, 397–405 (2013). https://doi.org/10.1016/j.electacta.2013.06.065
Wu, Q., Li, W., Liu, S., Jin, C.: Hydrothermal synthesis of N-doped spherical carbon from carboxymethylcellulose for CO2 capture. Appl. Surf. Sci. 369, 101–107 (2016). https://doi.org/10.1016/j.apsusc.2016.02.022
Yu, W., Wang, H., Liu, S., Mao, N., Liu, X., Shi, J., Liu, W., Chen, S., Wang, X.: N, O-codoped hierarchical porous carbons derived from algae for high-capacity supercapacitors and battery anodes. J. Mater. Chem. A. 4, 5973–5983 (2016). https://doi.org/10.1039/c6ta01821a
Xing, X., Jiang, W., Li, S., Zhang, X., Wang, W.: Preparation and analysis of straw activated carbon synergetic catalyzed by ZnCl2 -H3 PO4 through hydrothermal carbonization combined with ultrasonic assisted immersion pyrolysis. Waste Manag. 89, 64–72 (2019). https://doi.org/10.1016/j.wasman.2019.04.002
Zhang, W., Yu, C., Chang, L., Zhong, W., Yang, W.: Electrochimica acta three-dimensional nitrogen-doped hierarchical porous carbon derived from cross-linked lignin derivatives for high performance supercapacitors. Electrochim. Acta 282, 642–652 (2018). https://doi.org/10.1016/j.electacta.2018.06.100
Tan, J., Chen, H., Gao, Y., Li, H.: Nitrogen-doped porous carbon derived from citric acid and urea with outstanding supercapacitance performance. Electrochim. Acta 178, 144–152 (2015). https://doi.org/10.1016/j.electacta.2015.08.008
Titirici, M.M., Thomas, A., Antonietti, M.: Back in the black: hydrothermal carbonization of plant material as an efficient chemical process to treat the CO2 problem? New J Chem 31(6), 787–789 (2007)
Cui, X., Antonietti, M., Yu, S.H.: Structural effects of iron oxide nanoparticles and iron ions on the hydrothermal carbonization of starch and rice carbohydrates. Small. 2, 756–759 (2006). https://doi.org/10.1002/smll.200600047
Acknowledgements
The authors wish to thank supporting organizations, The Ministry of Agriculture, Food and Rural Affairs (OMAFRA) and the University of Guelph for ongoing HQP training support.
Funding
This work was funded by the Canadian Network for Research and Innovation in Machining Technology, Natural Sciences and Engineering Research Council of Canada (Grant No. RGPIN-2015-05093) and Biomass Canada of BioFuelNet Canada Network (Grant No. Project Number: ASC-16).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
MacDermid-Watts, K., Pradhan, R. & Dutta, A. Catalytic Hydrothermal Carbonization Treatment of Biomass for Enhanced Activated Carbon: A Review. Waste Biomass Valor 12, 2171–2186 (2021). https://doi.org/10.1007/s12649-020-01134-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01134-x