Abstract
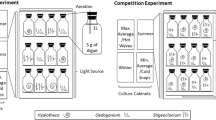
The Western Antarctic Peninsula (WAP) is a hot spot of global warming, including decreased sea-ice cover during winter and increased sedimentation during summer due to glacial melt. Subsequently, an altered irradiance and temperature regime in the water column may affect the performance of primary producers and change competitive structures. The brown, subtidal macroalgae Desmarestia menziesii and D. anceps are ecosystem engineers and of extreme importance for the Antarctic coastal ecosystem. Individuals of both species were collected from the field during the austral summer and exposed in two experiments to different temperatures (2 and 7 °C) or different irradiances (high and low) in combination with co-culturing the two algal species together (two-factorial design). No temperature, irradiance or co-cultivation effects on growth rates of D. menziesii and D. anceps were detected, but effects were possibly masked by the very low growth rates. Both D. menziesii and D. anceps are season anticipators, showing highest growth in late winter/spring and a dormancy state during summer. Photosynthetic efficiency was usually higher at 2 °C and low irradiance conditions compared to 7 °C and high irradiance and no co-culturing effects were detected. Parameters derived from P–E curves (rETRmax, Ek and α) were higher in D. menziesii compared to D. anceps, reflecting zonation patterns in the field. Future multifactorial experiments, taking seasons and different life-stages into account, are particularly needed to elucidate year-round effects of global warming on macroalgal key species that form the energetic base of the Antarctic coastal food webs.



Similar content being viewed by others
References
Amsler CD, Rowley RJ, Laur DR, Quetin LB, Ross RM (1995) Vertical distribution of Antarctic peninsular macroalgae: cover, biomass and species composition. Phycologia 34(5):424–430
Barner AK, Hacker SD, Menge BA, Nielsen KJ (2016) The complex net effect of reciprocal interactions and recruitment facilitation maintains an intertidal kelp community. J Ecol 104:33–43
Barrera-Oro E, Moreira E, Seefeldt MA, Francione MV, Quartino ML (2018) The importance of macroalgae and associated amphipods in the selective benthic feeding of sister rockcod species Notothenia rossii and N coriiceps (Nototheniidae) in West Antarctica. Polar Biol 42(2):317–334
Bartsch I, Wiencke C, Bischof K, Buchholz CM, Buck BH, Eggert A, Feuerpfeil P, Hanelt D, Jacobsen S, Karez R, Karsten U, Molis M, Roleda MY, Schubert H, Schumann R, Valentin K, Weinberger F, Wiese J (2008) The genus Laminaria sensu lato: recent insights and developments. Eur J Phycol 43:1–86
Bartsch I, Wiencke C, Laepple C (2012) Global seaweed biogeography under a changing climate: the prospected effects of temperature. In: Wiencke C, Bischof K (eds), Seaweed biology: novel insights into ecophysiology, ecology and utilization. Heidelberg: Springer
Brouwer PEM (1996) Decomposition in situ of the sublittoral Antarctic macroalga Desmarestia anceps Montagne. Polar Biol 16:129–137
Campana GL, Zacher K, Deregibus D, Momo FR, Wiencke C, Quartino ML (2018) Long term succession of benthic algae in Antarctica: structural patterns and glacial impact. Polar Biol 41(2):377–396
Carlsen BP, Johnsen G, Berge J, Kuklinski P (2007) Biodiversity patterns of macro-epifauna on different lamina parts of Laminaria digitata and Saccharina latissima collected during spring and summer 2004 in Kongsfjorden, Svalbard. Polar Biol 30(7):939–943
Carpenter RC (1990) Competition among marine macroalgae: a physiological perspective. J Phycol 26:6–12
Clarke A, Murphy EJ, Meredith MP, King JC, Peck LS, Barnes DK, Smith RC (2006) Climate change and the marine ecosystem of the western Antarctic Peninsula. Philos Trans R Soc B 362(1477):149–166
Clark GF, Stark JS, Johnston EL, Runcie JW, Goldsworthy PM, Raymond B, Riddle MJ (2013) Light-driven tipping points in polar ecosystems. Glob Change Biol 19(12):3749–3761
Clayton MN (1994) Evolution of the Antarctic marine benthic algal flora. J Phycol 30:897–904
Chen B, Zou D, Jiang H (2015) Elevated CO2 exacerbates competition for growth and photosynthesis between Gracilaria lemaneiformis and Ulva lactuca. Aquaculture 443:49–55
Coelho SM, Rijstenbil JW, Brown MT (2000) Impacts of anthropogenic stresses on the early development stages of seaweeds. J Aquat Ecosyst Stress Recov 7:317–333
Cordone G, Marina TI, Salinas V, Doyle SR, Saravia LA, Momo FR (2018) Effects of macroalgae loss in an Antarctic marine food web: applying extinction thresholds to food web studies. Peer J 6:e5531
Cook AJ, Fox AJ, Vaughan DG, Ferrigno JG (2005) Retreating glacier fronts on the Antarctic Peninsula over the past half-century. Science 308(5721):541–544
Deregibus D, Scharf FK, Pasotti F, Ruiz Barlett E, Servetto N, Abele D (2015) IMCONet Research Areas Map of Potter Cove, King-George Island (I. 25 de Mayo), with links to maps. Instituto Antártico Argentino, Buenos Aires, Argentina. https://doi.org/10.1594/PANGAEA.853859.
Deregibus D, Quartino ML, Campana GL, Momo FR, Wiencke C, Zacher K (2016) Photosynthetic light requirements and vertical distribution of macroalgae in newly ice-free areas in Potter Cove, South Shetland Islands, Antarctica. Polar Biol 39(1):153–166
Drew EA, Hastings RM (1992) A year-round ecophysiological study of Himantothallus grandifolius (Desmarestiales, Phaeophyta) at Signy Island, Antarctica. Phycologia 31(3–4):262–277
Ducklow HW, Fraser WR, Meredith MP, Stammerjohn SE, Doney SC, Martinson DG, Sailley SF, Schofield OM, Steinberg DK, Venables HJ, Amsler CD (2013) West Antarctic Peninsula: an ice-dependent coastal marine ecosystem in transition. Oceanography 26:190–203
Gómez I, Wiencke C, Weykam G (1995) Seasonal photosynthetic characteristics of Ascoseira mirabilis (Ascoseirales, Phaeophyceae) from King George Island, Antarctica. Mar Biol 123(1):167–172
Gómez I, Wiencke C (1997) Seasonal growth and photosynthetic performance of the Antarctic macroalga Desmarestia menziesii (Phaeophyceae) cultivated under fluctuating Antarctic daylengths. Bot Acta 110(1):25–31
Gómez I, Wulff A, Roleda MY, Huovinen P, Karsten U, Quartino ML, Dunton K, Wiencke C (2009) Light and temperature demands of marine benthic microalgae and seaweeds in polar regions. Bot Mar 52:593–608
Gómez I, Navarro NP, Huovinen P (2019) Bio-optical and physiological patterns in Antarctic seaweeds: a functional trait based approach to characterize vertical zonation. Prog Ocean 174:17–27
Graiff A, Liesner D, Karsten U, Bartsch I (2015) Temperature tolerance of western Baltic Sea Fucus vesiculosus – growth, photosynthesis and survival. J Exp Mar Biol Ecol 471:8–16
Gutkowski R, Maleszewski S (1989) Seasonal changes of the photosynthetic capacity of the Antarctic macroalga Adenocystis utricularis (Bory) Skottsberg. Polar Biol 10(2):145–148
Hapter R, Wozniak B, Dobrowolski K (1983) Primary production in Ezcurra Inlet during the Antarctic summer of 1977/78. Oceanologia 15:175–184
Heinrich S (2016) Short term physiological response to light, UVR and temperature stress in Antarctic versus Arctic habitat structuring brown algae. Algological Stud 151(1):151–165
Huang YM, Amsler MO, McClintock JB, Amsler CD, Baker BJ (2007) Patterns of gammarid amphipod abundance and species composition associated with dominant subtidal macroalgae along the western Antarctic Peninsula. Polar Biol 30:1417–1430
IPCC (2018) Summary for Policymakers. In: Masson-Delmotte V, Zhai P, Pörtner HO, Roberts D, Skea J, Shukla PR, Pirani A, Moufouma-Okia W, Pean C, Pidcock R, Connors S, Matthews JBR, Chen Y, Zhou X, Gomis MI, Lonnoy E, Maycock T, Tignor M, Waterfield T (eds.) Global warming of 15°C An IPCC Special Report on the impacts of global warming of 15°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty World Meteorological Organization, Geneva, p 32
Jassby AD, Platt T (1976) Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol Oceanogr 21:540–547
Karsten U, Wulff A, Roleda MY, Müller R, Steinhoff FS, Fredersdorf J, Wiencke C (2009) Physiological responses of polar benthic algae to ultraviolet radiation. Bot Mar 52:639–654
Kain JM (1989) The seasons in the subtidal. Br Phycol J 24(3):203–215
Khailov KM (1976) The relationships between weight, length, age and intensity of photosynthesis and organotrophy of macrophytes in the Barents Sea. Bot Mar 19(6):335–340
Klöser H, Quartino ML, Wiencke C (1996) Distribution of macroalgae and macroalgal communities in gradients of physical conditions in Potter Cove, King George Island, Antarctica. Hydrobiologia 333(1):1–17
Lüder UH, Knoetzel J, Wiencke C (2001) Acclimation of photosynthesis and pigments to seasonally changing light conditions in the endemic Antarctic red macroalga Palmaria decipiens. Polar Biol 24(8):598–603
Lüder UH, Wiencke C, Knoetzel J (2002) Acclimation of photosynthesis and pigments during and after six months of darkness in Palmaria decipiens (Rhodophyta): a study to simulate Antarctic winter sea ice cover. J Phycol 38(5):904–913
Lüning K (1984) Temperature tolerance and biogeography of seaweeds: The marine algal flora of Helgoland (North Sea) as an example. Helgoländer Meeresuntersuchungen 38:305–317
Lüning K, tom Dieck I (1989). Environmental triggers in algal seasonality. Bot Mar 32:389–397.
Marina TI, Salinas V, Cordone G, Campana G, Moreira E, Deregibus D, Torre L, Sahade R, Tatián M, Barrera-Oro E, De Troch M, Doyle S, Quartino ML, Saravia LA, Momo FR (2018) The food web of Potter Cove (Antarctica): complexity, structure and function. Estuar Coast Shelf Sci 200:141–151
Meredith MP, King JC (2005) Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophys Res Lett 32(19):1–5
Morgan-Kiss RM, Priscu JC, Pocock T, Gudynaite-Savitch L, Huner NPA (2006) Adaptation and acclimation of photosynthetic microorganisms to permanently cold environments. Microbiol Mol Biol Rev 70:222–252
Müller R, Wiencke C, Bischof K, Krock B (2009) Zoospores of three Arctic Laminariales under different UV radiation and temperature conditions: exceptional spectral absorbance properties and lack of phlorotannin induction. Photochem Photobiol 85:970–977
Nabivailo YV, Titlyanov EA (2006) Competitive relationships in natural and artificial algal communities. Russ J Mar Biol 32:21–31
Nabivailo YV, Skriptsova AV, Titlyanov EA (2014) The interspecific relationships of seaweeds and their role in the formation of communities of Ahnfeltia tobuchiensis (Kanno et Matsubara, 1932) Makienko, 1970 (Rhodophyta). Russ J Mar Biol 40:344–353
Olson AM, Lubchenco J (1990) Competition in seaweeds: Linking plant traits to competitive outcomes. J Phycol 26:1–6
Provasoli L (1968) Media and prospects for the cultivation of marine algae. In: Watanabe A, Hattori A (eds) Cultures and Collections of Algae. Japan Society of Plant Physiology, Hakone, pp 63–75
Quartino ML, Klöser H, Schloss I, Wiencke C (2001) Biomass and associations of benthic marine macroalgae from the inner Potter Cove (King George Island, Antarctica) related to depth and substrate. Polar Biol 24:349–355
Quartino ML, Boraso de Zaixso AL (2008) Summer macroalgal biomass in Potter Cove, South Shetland Islands, Antarctica: its production and flux to the ecosystem. Polar Biol 31:281–294
Quartino ML, Deregibus D, Campana GL, Latorre GEJ, Momo FR (2013) Evidence of macroalgal colonization on newly ice-free areas following glacial retreat in Potter Cove (South Shetland Islands). Antarctica PLoS ONE 8(3):e58223
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at https://www.R-project.org/
Rautenberger R, Huovinen P, Gómez I (2015) Effects of increased seawater temperature on UV tolerance of Antarctic marine macroalgae. Mar Biol 162(5):1087–1097
Reed DC (1990) The effects of variable settlement and early competition on pattern of kelp recruitment. Ecology 71:776–787
Reichardt W (1987) Burial of Antarctic macro algal debris in bioturbated deep-sea sediments. Deep-Sea Res 34:1761–1770
Schloss IR, Ferreyra GA, Curtosi A, Klöser H, Mercuri G, Pinola E (1997) Factors governing phytoplankton and particulate matter variation in Potter Cove, King George Island (Antarctica). In: Battaglia B, Valencia J, Walton DWH (eds) Antarctic communities: species, structure and survival. Cambridge University Press, Cambridge, pp 135–141
Schloss IR, Ferreyra GA, Curtosi A (1998) Primary production and conditions for phytoplankton growth in Potter Cove, King George Island. In: Wiencke C, Ferreyra GA, Amtz W, Rinaldi C (eds): The Potter Cove Coastal Ecosystem, Antarctica. Rep Polar Res 299:67–73
Schloss IR, Ferreyra GA (2002) Primary production, light and vertical mixing in Potter Cove, a shallow bay in the maritime Antarctic. Polar Biol 25(1):41–48
Schloss IR, Ferreyra GA, Ruiz-Pino D (2002) Phytoplankton biomass in Antarctic shelf zones: a conceptual model based on Potter Cove, King George Island. J Mar Syst 36:129–143
Schloss IR, Abele D, Moreau S, Demers S, Bers AV, González O, Ferreyra GA (2012) Response of phytoplankton dynamics to 19-year (1991–2009) climate trends in Potter Cove (Antarctica). J Mar Syst 92:53–66
Schoenrock KM, Schram JB, Amsler CD, McClintock JB, Angus RA (2015) Climate change impacts on overstory Desmarestia spp. from the western Antarctic Peninsula. Mar Biol 162:377–389
Shea R, Chopin T (2007) Effects of germanium dioxide, an inhibitor of diatom growth, on the microscopic laboratory cultivation stage of the kelp Laminaria saccharina. J Appl Phycol 19(1):27–32
Traiger SB, Konar B (2017). Supply and survival: glacial melt imposes limitations at the kelp microscopic life stage. Bot Mar 60(6):603–617.
Turley C (2013) Ocean acidification. In: Noone KJ, Sumaila UR, Diaz RJ (eds) Managing ocean environments in changing climate: sustainability and economic perspectives. Elsevier, Amsterdam, pp 15–44
Turner J, Bindschadler R, Convey P, Prisco G, Fahrbach E, Gutt J, Hodgson D, Mayewski P, Summerhayes C (eds) (2009) Antarctic climate change and the environment. Scientific Comittee on Antarctic Research, Cambridge
Turner J, Lu H, White I, King JC, Phillips T, Hosking JS, Bracegirdle TJ, Marshall GJ, Mulvaney R, Deb P (2016) Absence of 21st century warming on Antarctic Peninsula consistent with natural variability. Nature 535:411–415
Weykam G, Wiencke C (1996) Seasonal photosynthetic performance of the endemic Antarctic red alga Palmaria decipiens (Reinsch) Ricker. Polar Biol 16(5):357–361
Weykam G, Gómez I, Wiencke C, Iken K, Klöser H (1996) Photosynthetic characteristics and C: N ratios of macroalgae from King George Island (Antarctica). J Exp Mar Biol Ecol 204(1–2):1–22
Weykam G, Thomas DN, Wiencke C (1997) Growth and photosynthesis of the Antarctic red algae Palmaria decipiens (Palmariales) and Iridaea cordata (Gigartinales) during and following extended periods of darkness. Phycologia 36(5):395–405
Wiencke C (1990) Seasonality of brown macroalgae from Antarctica—a long-term culture study under fluctuating Antarctic daylengths. Polar Biol 10(8):589–600
Wiencke C, tom Dieck I (1989) Temperature requirements for growth and temperature tolerance of macroalgae endemic to the Antarctic region. Mar Ecol Prog Ser 54:189–197
Wiencke C, tom Dieck I (1990) Temperature requirements for growth and survival of macroalgae from Antarctica and southern Chile. Mar Ecol Progr Ser 59: 157–170
Wiencke C, Rahmel J, Karsten U, Weykam G, Kirst GO (1993) Photosynthesis of marine macroalgae from Antarctica: light and temperature requirements. Bot Acta 106(1):78–87
Wiencke C, Roleda MY, Gruber A, Clayton MN, Bischof K (2006) Susceptibility of zoospores to UV radiation determines upper depth distribution limit of Arctic kelps: evidence through field experiments. J Ecol 94(2):455–463
Wiencke C, Gómez I, Dunton K (2009) Phenology and seasonal physiological performance of polar seaweeds. Bot Mar 52(6):585–592.
Wiencke C, Amsler CD, Clayton MN (2014) Macroalgae. In: De Broyer C, Koubbi P, Griffiths HJ, Raymond B, Udekem d’Acoz, C d’,Van de Putte AP, Danis B, David B, Grant S, Gutt J, Held C, Hosie G. Huettmann F, Post A, Ropert-Coudert Y (eds.) Biogeographic atlas of the Southern Ocean. Scientific Committee on the Antarctic Research. Cambridge, pp 66–73.
Xu D, Li F, Gao Z, Wang D, Zhang X, Ye N, Zhuang Z (2013) Facilitative interactions between the green-tide macroalga Monostroma arctium and the red macroalga Porphyra yezoensis. J Exp Mar Biol Ecol 444:8–15
Zacher K, Savaglia V, Bartsch I (2016) Effects of temperature and interspecific competition on growth and photosynthesis of two endemic Antarctic Desmarestia species. Algol Stud 151(1):103–122
Acknowledgements
We would like to thank the diving and logistic team at Dallmann/ Carlini Sation. This work was supported by the Deutsche Forschungsgemeinschaft (DFG) in the framework of the priority programme "Antarctic Research with comparative investigations in Arctic ice areas" by a Grant Za735/1-1, by the Alfred-Wegener-Institute Helmholtz Centre for Polar and Marine Research and the international Research Network IMCONet funded by the Marie Curie Action IRSES IMCONet (FP7 IRSES - International Research Staff Exchange Scheme, Action No. 319718). We thank also Jilda Caccavo and Dina Brode-Roger for suggestions on language aspects and Dieter Piepenburg and the three anonymous reviewers for the helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Savaglia, V., Matula, C.V., Quartino, M.L. et al. Physiological response to irradiance, temperature and co-cultivation in Antarctic engineering brown algae (Desmarestia menziesii and D. anceps). Polar Biol 42, 2031–2044 (2019). https://doi.org/10.1007/s00300-019-02578-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-019-02578-1